Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods
Abstract
:1. Introduction
2. Materials and Methods
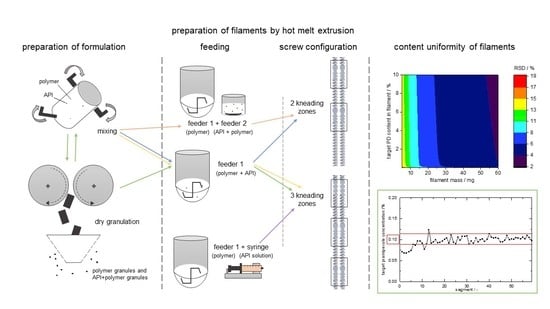
2.1. Experimental Procedure of Hot Melt Extrusion Runs
2.1.1. Split Feeding Setup
2.1.2. Liquid Feeding Setup
2.1.3. Granule Feeding Setup
2.2. Sample Preparation and HPLC Measurements
2.3. Production of Melts
2.4. Differential Scanning Calorimetry of Pramipexole
2.5. Mercury Porosimetry
2.6. Laser Diffraction
2.7. High-Resolution Mass Spectrometry
2.8. Thermogravimetry
2.9. Theoretical Background of the Deviation from Target Concentration
3. Results
3.1. Pramipexole Degradation within the Hot Melt Extrusion Process
3.2. Equilibrated Conditions for Extrusion
3.3. Equilibrium Condition for Drug Content
3.4. Limitation for Deviations of Drug Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | active pharmaceutical ingredient(s) |
| AV | acceptance value |
| bPMMA | basic butylated methacrylate copolymer |
| CV | coefficient of variation |
| DSC | differential scanning calorimetry |
| DTG | derivative thermogravimetry |
| HME | hot melt extrusion |
| IQR | interquartile range |
| MS | mass spectrometry |
| P | pramipexole |
| PD | pramipexole dichloride |
| PDM | pramipexole dichloride monohydrate |
| PVA | polyvinyl alcohol |
| RSD | relative standard deviation |
| SD | standard deviation |
References
- Dooley, M.; Markham, A. Pramipexole. Drugs Aging 1998, 12, 495–514. [Google Scholar] [CrossRef]
- Gültekin, H.E.; Serdar, T.; Füsun, A. An effective technology for the development of immediate release solid dosage forms containing low-dose drug: Fused deposition modeling 3D printing. Pharm. Res. 2019, 36, 1–13. [Google Scholar] [CrossRef]
- Zheng, J. (Ed.) Formulation and Analytical Development for Low-Dose Oral Drug Products; John Wiley & Sons: Hoboken, NJ, USA, 2009; p. 10. [Google Scholar]
- European Pharmacopoeia Commission. 2.9.40. Uniformity of dosage units. In European Pharmacopoeia; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2020; p. 3118. [Google Scholar]
- Ganderton, D.; Hunter, B.M. A comparison of granules prepared by pan granulation and by massing and screening. J. Pharm. Pharmacol. 1971, 23, 1S–10S. [Google Scholar] [CrossRef] [PubMed]
- Hersey, J.A. Ordered mixing: A new concept in powder mixing practice. Powder Technol. 1975, 11, 41–44. [Google Scholar] [CrossRef]
- Tian, Y.; Jones, D.S.; Donnelly, C.; Brannigan, T.; Li, S.; Andrews, G.P. A new method of constructing a drug–polymer temperature–composition phase diagram using hot-melt extrusion. Mol. Pharm. 2017, 15, 1379–1391. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-melt extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Loreti, G.; Maroni, A.; Dorly del Curto, M.; Melocchi, A.; Gazzaniga, A.; Zema, L. Evaluation of hot-melt extrusion technique in the preparation of HPC matrices for prolonged release. J. Pharm. Sci. 2014, 52, 77–85. [Google Scholar] [CrossRef]
- Lagan, C.; Huckle, J.E.; Katz, J.M.; Khorsand, B.; Daurio, D.; Andrews, G.P.; Chung, J.; Alvarez-Nunez, F. Hot Melt Extrusion of a Thermally Labile, High Melting Point Compound. AAPS PharmSciTech 2021, 22, 235. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, L.A.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Crystallization of amorphous solid dispersions of resveratrol during preparation and storage—Impact of different polymers. J. Pharm. Sci. 2013, 102, 171–184. [Google Scholar] [CrossRef]
- Llusa, M.; Mohr, S.; Baumgartner, R.; Paudel, A.; Koscher, G.; Khinast, J.G. Continuous low-dose feeding of highly active pharmaceutical ingredients in hot-melt extrusion. Drug Dev. Ind. Phar. 2016, 42, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kang, C.Y.; Kang, W.S.; Choi, H.G.; Han, H.K.; Lee, B.J. New investigation of distribution imaging and content uniformity of very low dose drugs using hot-melt extrusion method. Int. J. Pharm. 2013, 458, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sacher, S.; Heindl, N.; Urich, J.A.A.; Kruisz, J.; Khinast, J.G. A solution for low-dose feeding in continuous pharmaceutical processes. Int. J. Pharm. 2020, 591, 119969. [Google Scholar] [CrossRef]
- Repka, M.A.; Gerding, T.G.; Repka, S.L.; McGinity, J.W. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev. Ind. Phar. 1999, 25, 625–633. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef]
- Wang, H.; Dumpa, N.; Bandari, S.; Durig, T.; Repka, M.A. Fabrication of Taste-Masked Donut-Shaped Tablets Via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS PharmSciTech 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Martin, C. Twin screw extrusion for pharmaceutical processes. In Melt Extrusion; Springer: New York, NY, USA, 2013; pp. 47–79. [Google Scholar]
- Alshahrani, S.M.; Morott, J.T.; Alshetaili, A.S.; Tiwari, R.V.; Majumdar, S.; Repka, M.A. Influence of degassing on hot-melt extrusion process. Eur. J. Pharm. Sci. 2015, 80, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Gentzler, M.; Michaels, J.N.; Tardos, G.I. Quantification of segregation potential for polydisperse, cohesive, multi-component powders and prediction of tablet die-filling performance—A methodology for practical testing, re-formulation and process design. Powder Technol. 2015, 285, 96–102. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; Williams, A. Quantifying Uncertainty in Analytical Measurement; Eurachem/Citac: Teddington, UK, 2012. [Google Scholar]
- Anglov, T.; Byrialsen, K.; Carstensen, J.K.; Christensen, F.; Christensen, S.; Madsen, B.S.; Sørensen, E.; Sørensen, J.N.; Toftegård, K.; Winther, H.; et al. Uncertainty budget for final assay of a pharmaceutical product based on RP–HPLC. Accredit. Qual. Assur. 2003, 8, 225–230. [Google Scholar] [CrossRef]
- Hanson, J. Control of a system of loss-in-weight feeders for drug product continuous manufacturing. Powder Technol. 2018, 331, 236–243. [Google Scholar] [CrossRef]
- Egermann, H.; Frank, P. Novel approach to estimate quality of binary random powder mixtures: Samples of constant volume. I: Derivation of equation. J. Pharm. Sci. 1992, 81, 551–555. [Google Scholar] [CrossRef]
- Hermes, M. Kindgerechte, Niedrigdosierte Zubereitungen Mit Enalaprilmaleat. Ph.D. Thesis, HHU Düsseldorf, Göttingen, Germany, 2012. [Google Scholar]
- European Pharmacopeia. 8.0, Pramipexole Dihydrochloride Monohydrate. In European Pharmacopoeia; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2014. [Google Scholar]
- Wahl, P.R.; Treffer, D.; Mohr, S.; Roblegg, E.; Koscher, G.; Khinast, J.G. Inline monitoring and a PAT strategy for pharmaceutical hot melt extrusion. Int. J. Pharm. 2013, 455, 159–168. [Google Scholar] [CrossRef]
- Peng, F.; Zhao, Z.; Xia, X.; Cakmak, M.; Vogt, B.D. Enhanced impact resistance of three-dimensional-printed parts with structured filaments. ACS Appl. Mater. Interfaces 2018, 10, 16087–16094. [Google Scholar] [CrossRef] [PubMed]
- Wesholowski, J.; Prill, S.; Berghaus, A.; Thommes, M. Inline UV/Vis spectroscopy as PAT tool for hot-melt extrusion. Drug Deliv. Transl. Res. 2018, 8, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Predicting Drug Release from 3D Printed Oral Medicines Based on the Surface Area to Volume Ratio of Tablet Geometry. Pharmaceutics 2021, 13, 1453. [Google Scholar] [CrossRef]







| API and Excipients | Function | Manufacturer/Source |
|---|---|---|
| Pramipexole 2 HCl·H2O (PDM) | API | Chr. Olesen, Gentofte, Denmark |
| Polyvinyl alcohol (PVA) | matrix | Parteck MXP®, Merck, Darmstadt, Germany |
| Basic butylated methacrylate Copolymer (bPMMA) | matrix | Eudragit E PO®, Evonik, Essen, Germany |
| Fumed silica | glidant | Aerosil® 200 VV Pharma, Evonik, Essen, Germany |
| Mannitol | plasticizer | Parteck M®, Merck, Darmstadt, Germany |
| Sorbitol | plasticizer | Parteck SI® 200, Merck, Darmstadt, Germany |
| Temperature Profile in Zone 2–10/°C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zone | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| bPMMA-P (“physical mixture”) | 30 | 100 | 180 | 180 | 180 | 180 | 180 | 195 | 195 |
| PVA-P (“physical mixture”) | 30 | 180 | 190 | 200 | 220 | 220 | 220 | 220 | 220 |
| PVA-P (“3 kneading zones”) | 30 | 180 | 190 | 200 | 220 | 220 | 220 | 220 | 220 |
| PVA-P (“split feeding”) | 30 | 180 | 190 | 200 | 220 | 220 | 220 | 220 | 220 |
| PVA-P (“granule feeding”) | 120 | 180 | 190 | 195 | 200 | 200 | 215 | 220 | 220 |
| PVA-P (“liquid feeding”) | 20 | 20 | 65 | 170 | 205 | 205 | 205 | 205 | 205 |
| Screw Configuration (die–gear) * | |||||||||
| bPMMA-P and PVA-P (“physical mixtures”) | die–10 CE 1 L/D–KZ 1: 5 × 60°–3 × 30°–5 CE 1 L/D–KZ 2: 4 × 90°–5 × 60°–3 × 30°–16 CE 1 L/D–2 CE 3/2 L/D–1 L/D adapter–gear | ||||||||
| PVA-P (“3 kneading zones”) | die–10 CE 1 L/D–KZ 1: 4 × 90°–3 × 60°–3 × 30°–5 CE 1 L/D–KZ 2: 4 × 90°–3 × 60°–3 × 30°–5 CE 1 L/D–KZ 3: 10 × 60°–8 CE 1 L/D–1 CE ½ L/D–2 CE 3/2 L/D–1 L/D adapter–gear | ||||||||
| PVA-P (“split feeding”) | die–8 CE 1 L/D–KZ 1: 4 × 90°–4 × 60°–4 × 30°–5 CE 1 L/D–KZ 2: 5 × 90°–4 × 60°–3 × 30°–6 CE 1 L/D–3 DE L/D–3 CE 1 L/D–4 CE 3/2 L/D–1 L/D adapter–gear | ||||||||
| PVA-P (“granule feeding”) | die–10 CE 1 L/D–KZ 1: 6 × 60°–5 CE 1 L/D–KZ 2: 6 × 60°–6 CE 1 L/D–KZ 3: 4 × 90°–2 × 60°–2 × 30°–10 CE 1 L/D–2 CE 3/2 L/D–1 L/D adapter–gear | ||||||||
| PVA-P (“liquid feeding”) | die–10 CE 1 L/D–KZ 1: 5 × 60°–2 CE 1 L/D–2 CE LP 1 L/D–KZ 2: 5 × 60°–7 CE 1 L/D–KZ 3: 4 × 90°–3 × 60°–3 × 30°–10 CE 1 L/D–2 CE 3/2 L/D–1 L/D adapter–gear | ||||||||
| PVA | Pramipexole Dichloride | Fumed Silica | |
|---|---|---|---|
| Formulation 1 | 100% | - | - |
| Formulation 2 | 98% | 1% | 1% |
| Diameter | Diameter (x) | Diameter (y) | Ovality (dx–dy) | |||||
|---|---|---|---|---|---|---|---|---|
| Extrusion Setup | IQR1–99 | CV | IQR1–99 | CV | IQR1–99 | CV | IQR1–99 | CV |
| bPMMA-P (“physical mixture”) | 0.152 | 1.82 | 0.155 | 1.80 | 0.151 | 1.85 | 0.034 | 6.69 |
| PVA-P (“physical mixture”) | 0.582 | 8.72 | 0.582 | 1.57 | 0.582 | 8.72 | 0.022 | 21.23 |
| PVA-P (“3 kneading zones”) | 0.252 | 2.76 | 0.256 | 2.78 | 0.251 | 2.75 | 0.017 | 14.42 |
| PVA-P (“split feeding”) | 0.253 | 1.77 | 0.255 | 1.79 | 0.253 | 2.81 | 0.03 | 26.72 |
| PVA-P (“granules”) | 0.167 | 2.03 | 0.179 | 1.81 | 0.159 | 1.99 | 0.043 | 23.87 |
| PVA-P (“liquid feeding”) | 0.105 | 1.23 | 0.118 | 1.38 | 0.096 | 1.24 | 0.037 | 15.89 |
| Mean Content/% | SD/% | Content Uniformity (RSD)/% | Acceptance Value | |
|---|---|---|---|---|
| PVA-P (“physical mixture”) | 0.098 | 0.011 | 10.76 | 21.6 |
| PVA-P (“3 kneading zones”) | 0.099 | 0.008 | 8.28 | 16.4 |
| PVA-P (“split feeding”) | 0.091 | 0.010 | 11.21 | 27.9 |
| PVA-P (“granules”) | 0.101 | 0.006 | 6.00 | 12.2 |
| PVA-P (“liquid feeding”) | 0.096 | 0.006 | 6.35 | 14.7 |
| PDM | PVA | |
|---|---|---|
| Density (at 0.4 MPa) | 1.2343 g/cm3 | 1.2321 g/cm3 |
| Particle size | Dx10 6.0 µm Dx50 22.2 µm Dx90 68.1 µm | Dx10 11.8 µm Dx50 42.7 µm Dx90 96.7 µm |
| Filament Mass | RSD | Calculated RSD by Stange–Poole Equation | |
|---|---|---|---|
| 5% (w/w) filament | 10 mg | 8.95% | 8–11% |
| 20 mg | 8.00% | 6–8% | |
| 30 mg | 3.57% | 4–6% | |
| 50 mg | 4.35% | 4–6% | |
| 1% (w/w) filament | 10 mg | 9.45% | 8–11% |
| 20 mg | 5.05% | 6–8% | |
| 30 mg | 3.19% | 4–6% | |
| 50 mg | 3.55% | 4–6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamberlain, R.; Windolf, H.; Geissler, S.; Quodbach, J.; Breitkreutz, J. Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods. Pharmaceutics 2022, 14, 216. https://doi.org/10.3390/pharmaceutics14010216
Chamberlain R, Windolf H, Geissler S, Quodbach J, Breitkreutz J. Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods. Pharmaceutics. 2022; 14(1):216. https://doi.org/10.3390/pharmaceutics14010216
Chicago/Turabian StyleChamberlain, Rebecca, Hellen Windolf, Simon Geissler, Julian Quodbach, and Jörg Breitkreutz. 2022. "Precise Dosing of Pramipexole for Low-Dosed Filament Production by Hot Melt Extrusion Applying Various Feeding Methods" Pharmaceutics 14, no. 1: 216. https://doi.org/10.3390/pharmaceutics14010216