Skin Regenerative Potential of Cupuaçu Seed Extract (Theobroma grandiflorum), a Native Fruit from the Amazon: Development of a Topical Formulation Based on Chitosan-Coated Nanocapsules
Abstract
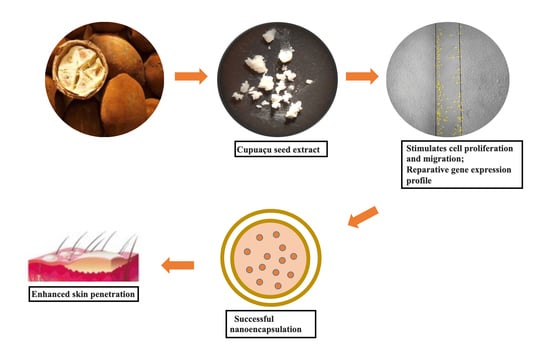
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Obtainment and Characterization of Cupuaçu Seed Extracts
2.3. In Vitro Biological Analyses
2.3.1. Cell Viability Assay
2.3.2. Proliferation Assay
2.3.3. Scratch Assay
2.3.4. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.4. Nanocapsule Preparation and Characterization
2.5. Mucoadhesion Assay
2.6. Stability
2.7. Irritation Assay
2.8. Skin Penetration
2.9. Biomarker Quantification
2.10. Data Analysis
3. Results and Discussion
3.1. Characterization of Cupuaçu Seed Extracts
3.2. In Vitro Biological Activity
3.2.1. Cell Viability and Proliferation
3.2.2. Cell Migration
3.2.3. Gene Expression
3.3. Nanocapsules Production and Characterization
3.4. Mucoadhesive Assay
3.5. Stability
3.6. Irritation Assay
3.7. Skin Penetration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Souza, J.M.L.; Rocha, J.M.; Cartaxo, C.B.C.; Vasconcelos, M.A.M.; Alvares, V.S.; Nascimento, M.M.; Yomura, R.T.B.; Kaefer, S. Monitoring and Optimization of Cupuaçu Seed Fermentation, Drying and Storage Processes. Microorganisms 2020, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, I.M.; Machado, F.L.; Silva, C.C.; Duvoisin, S.; Takeno, M.L.; Maia, P.J.D.; Manzato, L.; de Freitas, F.A. Application of calcined waste Cupuaçu (Theobroma grandiflorum) seeds as a low-cost solid catalyst in soybean oil ethanolysis: Statistical optimization. Energy Convers. Manag. 2019, 200, 112095. [Google Scholar] [CrossRef]
- De Azevedo, A.B.A.; Kopcak, U.; Mohamed, R.S. Extraction of fat from fermented Cupuaçu seeds with supercritical solvents. J. Supercrit. Fluids 2003, 27, 223–237. [Google Scholar] [CrossRef]
- Da Silva, W.G.; Baruffaldi, R.; Oliveira, M.N.; Fedeli, E. The fat of Theobroma grandiflorum “Cupuaçu” seeds—Substitute for cocoa-butter. Ind. Aliment. 1999, 38, 546. [Google Scholar]
- Oliveira, S.C.; Ramalho, S.A.; Gualberto, N.C.; Gomes, E.D.; Miranda, R.M.; Narain, N. Ascorbic acid content, phenolic compounds and antioxidant capacity of Brazilian exotic fruits acai (Euterpe oleraceae Mart.) and Cupuaçu (Theobroma grandiflorum Schum.). Planta Med. 2011, 77, 1412. [Google Scholar] [CrossRef]
- Punaro, G.R.; Lima, D.Y.; Rodrigues, A.M.; Pugliero, S.; Mouro, M.G.; Rogero, M.M.; Higa, E.M.S. Cupuaçu extract reduces nitrosative stress and modulates inflammatory mediators in the kidneys of experimental diabetes. Clin. Nutr. 2019, 38, 364–371. [Google Scholar] [CrossRef]
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The mechanism of skin lipids influencing skin status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.; Favoreto, S.; Oliveira, L.L.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic acid modulation of the immune response in wound healing: A new approach for skin repair. Immunobiology 2011, 216, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Souza, M.A.; Ferro, E.A.; Favoreto, S.; Pena, J.D., Jr. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004, 12, 235–243. [Google Scholar] [CrossRef]
- Rodrigues, H.G.; Vinolo, M.A.R.; Magdalon, J.; Vitzel, K.; Nachbar, R.T.; Pessoa, A.F.M.; dos Santos, M.F.; Hatanaka, E.; Calder, P.C.; Curi, R. Oral Administration of Oleic or Linoleic Acid Accelerates the Inflammatory Phase of Wound Healing. J. Investig. Dermatol. 2012, 132, 208–215. [Google Scholar] [CrossRef]
- Cordenonsi, L.M.; Faccendini, A.; Catanzaro, M.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Raffin, R.P.; Schapoval, E.E.S.; Lanni, C.; Sandri, G.; et al. The role of chitosan as coating material for nanostructured lipid carriers for skin delivery of fucoxanthin. Int. J. Pharm. 2019, 567, 118487. [Google Scholar] [CrossRef]
- Ushirobira, C.Y.; Afiune, L.A.F.; Pereira, M.N.; Cunha-Filho, M.; Gelfuso, G.M.; Gratieri, T. Dutasteride nanocapsules for hair follicle targeting: Effect of chitosan-coating and physical stimulus. Int. J. Biol. Macromol. 2020, 151, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Polymeric nanocapsules: A review on design and production methods for pharmaceutical purpose. Methods 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, G.; Vigani, B.; Rossi, S.; Sandri, G.; Mele, E. Chitosan-Coated Poly(lactic acid) Nanofibres Loaded with Essential Oils for Wound Healing. Polymers 2021, 13, 2582. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.S.; Oliveira, P.M.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Latanoprost Loaded in Polymeric Nanocapsules for Effective Topical Treatment of Alopecia. AAPS PharmSciTech 2020, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Validi, M.; Gholipour, A.; Makvandi, P.; Sharifi, E. Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioeng. Transl. Med. 2021, e10254. [Google Scholar] [CrossRef]
- Matos, B.N.; Pereira, M.N.; Bravo, M.D.O.; Filho, M.C.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles loading oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. Int. J. Biol. Macromol. 2019, 154, 1265–1275. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E. Topical drug delivery using chitosan nano- and microparticles. Expert Opin. Drug Deliv. 2012, 9, 1129–1146. [Google Scholar] [CrossRef]
- Dodane, V. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef]
- Lias, S.; Mikaia, A.; Sparkman, O.; Stein, S.; Zaikin, V. The NIST/EPA/NIH mass spectral database: Simultaneous control of quality and quantity. In Proceedings of the 45th ASMS Conference on Mass Spectrometry and Allied Topics, Palm Springs, CA, USA, 1–5 June 1997. [Google Scholar]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.A.; Adorne, M.D.; Colomé, L.M.; Abdalla, D.S.; Guterres, S.S.; Pohlmann, A.R. Hemocompatibility of poly(ε-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int. J. Pharm 2012, 426, 271–279. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Pereira, M.N.; Tolentino, S.; Pires, F.Q.; Anjos, J.L.; Alonso, A.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Nanostructured lipid carriers for hair follicle-targeted delivery of clindamycin and rifampicin to hidradenitis suppurativa treatment. Colloids Surf. B Biointerfaces 2020, 197, 111448. [Google Scholar] [CrossRef] [PubMed]
- Angelo, T.; Barbalho, G.N.; Gelfuso, G.; Gratieri, T. Minoxidil topical treatment may be more efficient if applied on damp scalp in comparison with dry scalp. Dermatol. Ther. 2016, 29, 330–333. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Matos, B.N.; Santo, M.E.L.E.; Silva, V.R.; Chaves, S.B.; Gelfuso, G.M.; Cunha-Filho, M.; Gratieri, T. In vitro skin model for the evaluation of burn healing drug delivery systems. J. Drug Deliv. Sci. Technol. 2021, 62, 102330. [Google Scholar] [CrossRef]
- Bannon, C.D.; Green, G.J.; Craske, J.D.; Hai, N.T.; Harper, N.L.; O.′rourke, K.L. Analysis of fatty acid methyl esters with high accuracy and reliability. III: Literature review of and investigations into the development of rapid procedures for the methoxide-catalysed methanol of fats and oils. J. Chromatogr. 1982, 247, 63. [Google Scholar] [CrossRef]
- Vasconcelos, M.N.L.; Silva, M.L.; Maia, J.G.S.; Gottlieb, O.R. Estudo químico das sementes do Cupuaçu. Acta Amaz. 1975, 5, 293–295. [Google Scholar] [CrossRef]
- Krist, S. Cupuacu Butter. In Vegetable Fats and Oils; Springer: Cham, Germany, 2020. [Google Scholar] [CrossRef]
- Sakar, M.S.; Eyckmans, J.; Pieters, R.; Eberli, D.; Nelson, B.J.; Chen, C. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat. Commun. 2016, 7, 11036. [Google Scholar] [CrossRef] [Green Version]
- Ichim, T.E.; O’Heeron, P.; Kesari, S. Fibroblasts as a practical alternative to mesenchymal stem cells. J. Transl. Med. 2018, 16, 212. [Google Scholar] [CrossRef] [Green Version]
- Alencar-Silva, T.; Zonari, A.; Foyt, D.; Gang, M.; Pogue, R.; Saldanha-Araujo, F.; Dias, S.C.; Franco, O.L.; Carvalho, J.L. IDR-1018 induces cell proliferation, migration, and reparative gene expression in 2D culture and 3D human skin equivalents. J. Tissue Eng. Regen. Med. 2019, 13, 2018–2030. [Google Scholar] [CrossRef]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin signaling in wound repair. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 248–257. [Google Scholar] [CrossRef]
- Supp, D.M.; Hahn, J.M.; McFarland, K.L.; Glaser, K. Inhibition of Hyaluronan Synthase 2 Reduces the Abnormal Migration Rate of Keloid Keratinocytes. J. Burn. Care Res. 2014, 35, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Du, Y.; Shen, Y.; He, Y.; Zhao, H.; Li, Z. TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front. Biosci. 2012, 17, 2667–2674. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.Y.; El-Baz, L.M.; House, S.L.; Cilvik, S.; Dorry, S.J.; Shoukry, N.M.; Salem, M.L.; Hafez, H.S.; Dulin, N.O.; Ornitz, D.M.; et al. Fibroblast growth factor 2 decreases bleomycin-induced pulmonary fibrosis and inhibits fibroblast collagen production and myofibroblast differentiation. J. Pathol. 2018, 246, 54–66. [Google Scholar] [CrossRef]
- Dolivo, D.; Larson, S.; Dominko, T. Fibroblast Growth Factor 2 as an Antifibrotic: Antagonism of Myofibroblast Differentiation and Suppression of Pro-Fibrotic Gene Expression. Cytokine Growth Factor Rev. 2017, 38, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.J.; Longaker, M.T.; Lorenz, H.P. Scarless Fetal Wound Healing: A Basic Science Review. Plast. Reconstr. Surg. 2010, 126, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional Biomaterials for Treatment of Chronic Wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- Tolentino, S.; Pereira, M.N.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Targeted clindamycin delivery to pilosebaceous units by chitosan or hyaluronic acid nanoparticles for improved topical treatment of acne vulgaris. Carbohydr. Polym. 2020, 253, 117295. [Google Scholar] [CrossRef]
- Han, H.-K.; Jung, I.-W.; Lee, B.-J. Effective mucoadhesive liposomal delivery system for risedronate: Preparation and in vitro/in vivo characterization. Int. J. Nanomed. 2014, 9, 2299–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Yasrebi, N.; Zarmi, A.H.; Larypoor, M.; Zeynali, M.; Ebrahimi-Hosseinzadeh, B.; Mokhtari-Hosseini, Z.B.; Alvandi, H. In vivo and in vitro evaluation of the wound healing properties of chitosan extracted from Trametes versicolor. J. Polym. Res. 2021, 28, 399. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Matos, R.S.; Lopes, G.A.C.; Ferreira, N.S.; Pinto, E.P.; Carvalho, J.C.T.; Figueiredo, S.S.; Oliveira, A.F.; Zamora, R.R.M. Superficial Characterization of Kefir Biofilms Associated with Açaí and Cupuaçu Extracts. Arab. J. Sci. Eng. 2017, 43, 3371–3379. [Google Scholar] [CrossRef]








| Compounds | Percentage in Extract (%) |
|---|---|
| Oleic acid | 41.81 |
| Stearic acid | 35.79 |
| Palmitic acid | 8.67 |
| Eicosanoic acid | 7.85 |
| Linoleic acid | 2.94 |
| Behenic acid | 1.09 |
| Methyl (11E)-11-icosenoate | 0.38 |
| Ethyl oleate | 0.28 |
| Myristic acid | 0.15 |
| Not identified | 0.48 |
| Total | 99.44 |
| Sample | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) | EE% | pH |
|---|---|---|---|---|---|
| NC | 278.3 ± 5.0 | 0.18 ± 0.02 | −26.2 ± 0.8 | 94.6 ± 0.7 | 5.5 |
| NC-CH | 337.2 ± 2.1 | 0.27 ± 0.01 | + 38.5 ± 0.9 | 94.4 ± 0.6 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalho, G.N.; Matos, B.N.; da Silva Brito, G.F.; da Cunha Miranda, T.; Alencar-Silva, T.; Sodré, F.F.; Gelfuso, G.M.; Cunha-Filho, M.; Carvalho, J.L.; da Silva, J.K.d.R.; et al. Skin Regenerative Potential of Cupuaçu Seed Extract (Theobroma grandiflorum), a Native Fruit from the Amazon: Development of a Topical Formulation Based on Chitosan-Coated Nanocapsules. Pharmaceutics 2022, 14, 207. https://doi.org/10.3390/pharmaceutics14010207
Barbalho GN, Matos BN, da Silva Brito GF, da Cunha Miranda T, Alencar-Silva T, Sodré FF, Gelfuso GM, Cunha-Filho M, Carvalho JL, da Silva JKdR, et al. Skin Regenerative Potential of Cupuaçu Seed Extract (Theobroma grandiflorum), a Native Fruit from the Amazon: Development of a Topical Formulation Based on Chitosan-Coated Nanocapsules. Pharmaceutics. 2022; 14(1):207. https://doi.org/10.3390/pharmaceutics14010207
Chicago/Turabian StyleBarbalho, Geisa Nascimento, Breno Noronha Matos, Gabriel Ferreira da Silva Brito, Thamires da Cunha Miranda, Thuany Alencar-Silva, Fernando Fabriz Sodré, Guilherme Martins Gelfuso, Marcilio Cunha-Filho, Juliana Lott Carvalho, Joyce Kelly do Rosário da Silva, and et al. 2022. "Skin Regenerative Potential of Cupuaçu Seed Extract (Theobroma grandiflorum), a Native Fruit from the Amazon: Development of a Topical Formulation Based on Chitosan-Coated Nanocapsules" Pharmaceutics 14, no. 1: 207. https://doi.org/10.3390/pharmaceutics14010207