Strong Antimicrobial Activity of Highly Stable Nanocomposite Containing AgNPs Based on Water-Soluble Triazole-Sulfonate Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Poly(VT-co-Na-VSA)
2.3. AgNPs Nanocomposite
2.4. Characterization
2.5. Antimicrobial Activity
3. Results and Discussion
3.1. Copolymerization of VT with Na-VSA
3.2. Synthesis of Nanocomposite
3.3. FTIR Characterization and X-Ray Diffraction
3.4. UV–Visible Spectroscopy and Electron Microscopy
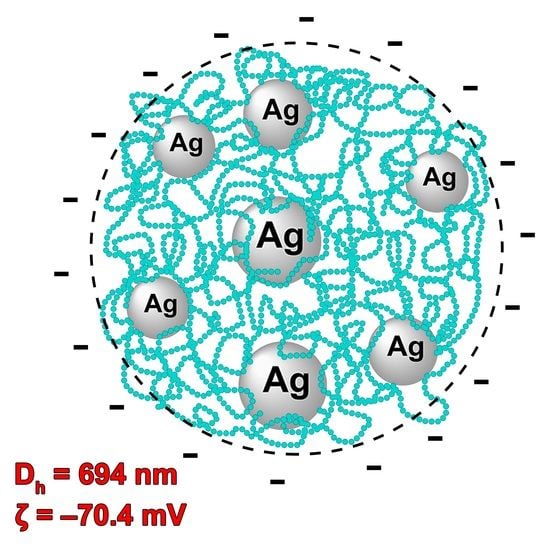
3.5. Dynamic and Electrophoretic Light Scattering
3.6. Thermogravimetric Analysis and Differential Scanning Calorimetry
3.7. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Lee, B.; Lee, M.J.; Yun, S.J.; Kim, K.; Choi, I.-H.; Park, S. Silver nanoparticles induce reactive oxygen species-mediated cell cycle delay and synergistic cytotoxicity with 3-bromopyruvate in Candida albicans, but not in Saccharomyces cerevisiae. Int. J. Nanomed. 2019, 14, 4801–4816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haggag, E.; Elshamy, A.; Rabeh, M.; Gabr, N.; Salem, M.; Youssif, K.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, M.; Rai, M. Recent progress in nanoformulations of silver nanoparticles with cellulose, chitosan, and alginic acid biopolymers for antibacterial applications. Appl. Microbiol. Biotechnol. 2019, 103, 8669–8676. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of Recent Studies Dealing with the Antibacterial Properties of Silver Nanoparticles: Fact and Opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef] [Green Version]
- Stojkovska, J.; Zvicer, J.; Obradovic, B. Preclinical functional characterization methods of nanocomposite hydrogels containing silver nanoparticles for biomedical applications. Appl. Microbiol. Biotechnol. 2020, 104, 4643–4658. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Saravanakumar, K.; Jin, T.; Wang, M.-H. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int. J. Nanomed. 2019, 14, 3427–3438. [Google Scholar] [CrossRef] [Green Version]
- Murugesan, K.; Koroth, J.; Srinivasan, P.P.; Singh, A.; Mukundan, S.; Karki, S.S.; Choudhary, B.; Gupta, C.M. Effects of green synthesised silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models [Corrigendum]. Int. J. Nanomed. 2019, 14, 6133–6134. [Google Scholar] [CrossRef] [Green Version]
- Gharpure, S.; Akash, A.; Ankamwar, B. A Review on Antimicrobial Properties of Metal Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 3303–3339. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Shin, H.-S. Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. Int. J. Nanomed. 2019, 14, 6679–6690. [Google Scholar] [CrossRef] [Green Version]
- Habibipour, R.; Moradi-Haghgou, L.; Farmany, A. Green synthesis of AgNPs@PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Int. J. Nanomed. 2019, 14, 6891–6899. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.A.; Mekhamer, W.K.; Merghani, N.M.; Hendi, A.A.; Ortashi, K.M.O.; Al-Abbas, F.; Eisa, N.E. Green Synthesis, Characterization, and Antibacterial Activity of Silver/Polystyrene Nanocomposite. J. Nanomater. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [Green Version]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of Silver Nanoparticles from Melia azedarach: Enhancement of Antibacterial, Wound Healing, Antidiabetic and Antioxidant Activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef] [Green Version]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of Silver Nanoparticles Using a Novel Cyanobacteria Desertifilum sp. extract: Their Antibacterial and Cytotoxicity Effects. Int. J. Nanomed. 2020, 15, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Krystosiak, P.; Tomaszewski, W.; Megiel, E. High-density polystyrene-grafted silver nanoparticles and their use in the preparation of nanocomposites with antibacterial properties. J. Colloid Interface Sci. 2017, 498, 9–21. [Google Scholar] [CrossRef]
- Shurygina, I.A.; Prozorova, G.F.; Trukhan, I.S.; Korzhova, S.A.; Fadeeva, T.V.; Pozdnyakov, A.S.; Dremina, N.N.; Emel’yanov, A.I.; Kuznetsova, N.P.; Shurygin, M.G. NonToxic Silver/Poly-1-Vinyl-1,2,4-Triazole Nanocomposite Materials with Antibacterial Activity. Nanomaterials 2020, 10, 1477. [Google Scholar] [CrossRef]
- Ermakova, T.G.; Shaulina, L.P.; Kuznetsova, N.P.; Volkova, L.I.; Pozdnyakov, A.S.; Prozorova, G.F. Sorption of noble metal compounds by cross-linked copolymer of 1-vinyl-1,2,4-triazole with acrylic acid. Russ. J. Appl. Chem. 2012, 85, 35–40. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Ivanova, A.A.; Emel’yanov, A.I.; Prozorova, G.F. Metal-polymer Ag nanocomposites based on hydrophilic nitrogen- and sulfur-containing copolymers: Control of nanoparticle size. Russ. Chem. Bull. 2020, 69, 715–720. [Google Scholar] [CrossRef]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B.; et al. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials. 2021, 14, 6915. [Google Scholar] [CrossRef]
- Zezin, A.A.; Klimov, D.I.; Zezina, E.A.; Mkrtchyan, K.V.; Feldman, V.I. Controlled radiation-chemical synthesis of metal polymer nanocomposites in the films of interpolyelectrolyte complexes: Principles, prospects and implications. Radiat. Phys. Chem. 2020, 169, 1–15. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Ivanova, A.A.; Emelyanov, A.I.; Ermakova, T.G.; Prozorova, G.F. Nanocomposites with silver nanoparticles based on copolymer of 1-vinyl-1,2,4-triazole with N-vinylpyrrolidone. Russ. Chem. Bull. 2017, 66, 1099–1103. [Google Scholar] [CrossRef]
- Demchenko, V.L.; Kobylinskyi, S.M.; Riabov, S.V.; Shtompel, V.I.; Iurzhenko, M.V.; Rybalchenko, N.P. Novel approach to the formation of silver-containing nanocomposites by thermochemical reduction of Ag+ ions in interpolyelectrolyte-metal complexes. Appl. Nanosci. 2020, 10. [Google Scholar] [CrossRef]
- Zezina, E.A.; Emel’yanov, A.I.; Pozdnyakov, A.S.; Prozorova, G.F.; Abramchuk, S.S.; Feldman, V.I.; Zezin, A.A. Radiation-induced synthesis of copper nanostructures in the films of interpolymer complexes. Radiat. Phys. Chem. 2019, 158, 115–121. [Google Scholar] [CrossRef]
- Mkrtchyan, K.V.; Zezin, A.A.; Zezina, E.A.; Abramchuk, S.S.; Baranova, I.A. Formation of metal nanostructures under X-ray radiation in films of interpolyelectrolyte complexes with different silver ion content. Russ. Chem. Bull. 2020, 69, 1731–1739. [Google Scholar] [CrossRef]
- Gorbunova, M.N.; Batueva, T.D.; Kiselkov, D.M.; Strelnikov, V.N. Silver nanocomposites based on copolymers of N,N-diallyl-N’-acetylhydrazine with N-vinylpyrrolidone. Russ. Chem. Bull. 2021, 70, 1706–1712. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Emel’yanov, A.I.; Korzhova, S.A.; Kuznetsova, N.P.; Bolgova, Y.I.; Trofimova, O.M.; Semenova, T.A.; Prozorova, G.F. Green Synthesis of Stable Nanocomposites Containing Copper Nanoparticles Incorporated in Poly-N-vinylimidazole. Polymers 2021, 13, 3212. [Google Scholar] [CrossRef]
- Salaheldin, H.I.; Almalki, M.H.K.; Hezma, A.E.M.; Osman, G.E.H. Facile synthesis of silver nanoparticles mediated by polyacrylamide-reduction approach to antibacterial application. IET Nanobiotechnol. 2017, 11, 448–453. [Google Scholar] [CrossRef]
- Qasim, M.; Udomluck, N.; Chang, J.; Park, H.; Kim, K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomed. 2018, 13, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Pozdnyakov, A.; Emel’yanov, A.; Kuznetsova, N.; Ermakova, T.; Bolgova, Y.; Trofimova, O.; Albanov, A.; Borodina, T.; Smirnov, V.; Prozorova, G. A Polymer Nanocomposite with CuNP Stabilized by 1-Vinyl-1,2,4-triazole and Acrylonitrile Copolymer. Synlett 2016, 27, 900–904. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, S.H.; Kim, W.G. MMA/MPEOMA/VSA copolymer as a novel blood-compatible material: Ex vivo platelet adhesion study. J. Mater. Sci. Mater. Med. 2004, 15, 155–159. [Google Scholar] [CrossRef]
- Kim, H., II.; Park, S.J.; Kim, S.I.; Kim, N.G.; Kim, S.J. Electroactive polymer hydrogels composed of polyacrylic acid and poly(vinyl sulfonic acid) copolymer for application of biomaterial. Synth. Met. 2005, 155, 674–676. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.; Cai, C.; Rytting, E.; Steele, T.; Kissel, T. Biodegradable Branched Polyesters Poly(vinyl sulfonate-covinyl alcohol)-graft Poly(D,L-lactic-coglycolic acid) as a Negatively Charged Polyelectrolyte Platform for Drug Delivery: Synthesis and Characterization. Macromolecules 2008, 41, 2791–2799. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, S.H. MMA/MPEOMA/VSA copolymer as a novel blood-compatible material: Effect of PEO and negatively charged side chains on protein adsorption and platelet adhesion. J. Biomed. Mater. Res. 2002, 60, 44–52. [Google Scholar] [CrossRef]
- Horbett, T.A. Selected aspects of the state of the art in biomaterials for cardiovascular applications. Colloids Surf. B Biointerfaces 2020, 191, 110986. [Google Scholar] [CrossRef]
- Murakami, D.; Segami, Y.; Ueda, T.; Tanaka, M. Control of interfacial structures and anti-platelet adhesion property of blood-compatible random copolymers. J. Biomater. Sci. Polym. Ed. 2020, 31, 207–218. [Google Scholar] [CrossRef]
- Tsai, B.-H.; Chuang, Y.-H.; Cheng, C.-H.; Lin, J.-C. Sulfonation and Characterization of Tert-Butyl Styrene/Styrene/Isoprene Copolymer and Polypropylene Blends for Blood Compatibility Applications. Polymers 2020, 12, 1351. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Ivanova, A.A.; Emel’yanov, A.I.; Bolgova, Y.I.; Trofimova, O.M.; Prozorova, G.F. Water-soluble stable polymer nanocomposites with AuNPs based on the functional poly(1-vinyl-1,2,4-triazole-co-N-vinylpyrrolidone). J. Organomet. Chem. 2020, 922, 121352. [Google Scholar] [CrossRef]
- Fisenko, V.P.; Arzamastsev, E.V.; Babayan, E.A. Guidance for Experimental (Pre-Clinical) Investigation of New Pharmacological Compounds; U.S. Department of Health and Human Services, Food and Drug Administration: Rockville, MD, USA, 2000; pp. 1–398. [Google Scholar]
- Mazyar, N.L.; Annenkov, V.V.; Kruglova, V.A.; Anan’ev, S.M.; Danilovtseva, E.N.; Rokhin, A.V.; Zinchenko, S. V Acid-base properties of poly(1-vinylazoles) in aqueous solution. Russ. Chem. Bull. 2000, 49, 2013–2017. [Google Scholar] [CrossRef]










| Microorganisms | MIC/MBC, μg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 500-32 | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | |
| Escherichia coli ATCC 25,922 | − −/− − | − −/− − | − −/− − | − −/− − | − −/− − | − −/− − | − − /+ + | + +/+ + | + +/+ + |
| Pseudomonas aeruginosa ATCC 27,853 | − −/− − | − −/− − | − −/− − | − −/− − | − −/− − | − −/− − | − −/− − | − − /+ + | + +/+ + |
| Klebsiella pneumoniae ATCC 700,603 (EBSL) | − −/− − | − −/− − | − −/− − | − − /+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
| Staphylococcus aureus ATCC 25,923 | − −/− − | − −/− − | − −/− − | − −/− − | − − /+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
| Enterococcus faecalis ATCC 29,212 | − −/− − | − −/− − | − −/− − | − − /+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
| Microorganisms | MIC/MBC, μg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 500-32 | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | |
| Escherichia coli ATCC 25,922 | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
| Pseudomonas aeruginosa ATCC 27,853 | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
| Klebsiella pneumoniae ATCC 700,603 (EBSL) | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
| Staphylococcus aureus ATCC 25,923 | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
| Enterococcus faecalis ATCC 29,212 | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + | + +/+ + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozdnyakov, A.; Emel’yanov, A.; Ivanova, A.; Kuznetsova, N.; Semenova, T.; Bolgova, Y.; Korzhova, S.; Trofimova, O.; Fadeeva, T.; Prozorova, G. Strong Antimicrobial Activity of Highly Stable Nanocomposite Containing AgNPs Based on Water-Soluble Triazole-Sulfonate Copolymer. Pharmaceutics 2022, 14, 206. https://doi.org/10.3390/pharmaceutics14010206
Pozdnyakov A, Emel’yanov A, Ivanova A, Kuznetsova N, Semenova T, Bolgova Y, Korzhova S, Trofimova O, Fadeeva T, Prozorova G. Strong Antimicrobial Activity of Highly Stable Nanocomposite Containing AgNPs Based on Water-Soluble Triazole-Sulfonate Copolymer. Pharmaceutics. 2022; 14(1):206. https://doi.org/10.3390/pharmaceutics14010206
Chicago/Turabian StylePozdnyakov, Alexander, Artem Emel’yanov, Anastasiya Ivanova, Nadezhda Kuznetsova, Tat’yana Semenova, Yuliya Bolgova, Svetlana Korzhova, Olga Trofimova, Tat’yana Fadeeva, and Galina Prozorova. 2022. "Strong Antimicrobial Activity of Highly Stable Nanocomposite Containing AgNPs Based on Water-Soluble Triazole-Sulfonate Copolymer" Pharmaceutics 14, no. 1: 206. https://doi.org/10.3390/pharmaceutics14010206