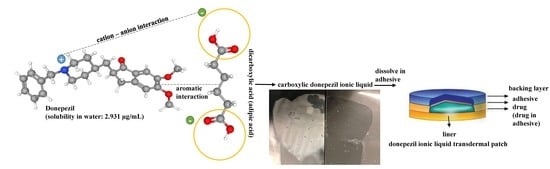
Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Donepezil (DPZ) Ionic Liquid (IL) Preliminary Study
2.2.2. Preparation of DPZ ILs
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Optical Microscopy
2.2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.6. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.2.7. Equilibrium Solubility Determination
2.2.8. In Vitro Skin Permeation Test
2.2.9. Acrylic Adhesive and DPZ IL Compatibility Test
2.2.10. Drug-In-Adhesive Patch Preparation
2.2.11. DPZ IL Patch Formulation Development
2.2.12. Ex Vivo Permeability Test
2.2.13. HPLC Analysis
2.2.14. Statistical Analysis
3. Results and Discussion
3.1. Donepezil Ionic Liquid (DPZ IL) Preliminary Study
3.2. Characterization of DPZ ILs
3.3. In Vitro Skin Permeation Tests
3.4. Acrylic Adhesive and DPZ IL Compatibility Test
3.5. DPZ IL Patch Formulation Preparation
3.6. Ex Vivo Skin Permeability Test
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Cummings, J.; Jack, C.R.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Loveman, E.; Green, C.; Kirby, J.; Takeda, A.; Picot, J.; Payne, E.; Clegg, A. The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease. Health Technol. Assess. 2006, 10, 1–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacabelos, R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr. Dis. Treat. 2007, 3, 303–333. [Google Scholar] [PubMed]
- Sozio, P.; Cerasa, L.S.; Marinelli, L.; Di Stefano, A. Transdermal donepezil on the treatment of Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2012, 8, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Liu, C.; Piao, H.; Quan, P.; Fang, L. Enhanced Drug Loading in the Drug-in-Adhesive Transdermal Patch Utilizing a Drug–Ionic Liquid Strategy: Insight into the Role of Ionic Hydrogen Bonding. Mol. Pharm. 2021, 18, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, S.; Yin, H.; Qi, M.; Hong, M.; Ren, G.-B. Development of gliclazide ionic liquid and the transdermal patches: An effective and noninvasive sustained release formulation to achieve hypoglycemic effects. Eur. J. Pharm. Sci. 2021, 164, 105915. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fang, F.; Zheng, L.; Ji, W.; Qi, M.; Hong, M.; Ren, G. Ionic liquid form of donepezil: Preparation, characterization and formulation development. J. Mol. Liq. 2020, 300, 112308. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Kundu, N.; Roy, S.; Mukherjee, D.; Maiti, T.K.; Sarkar, N. Unveiling the Interaction between Fatty-Acid-Modified Membrane and Hydrophilic Imidazolium-Based Ionic Liquid: Understanding the Mechanism of Ionic Liquid Cytotoxicity. J. Phys. Chem. B 2017, 121, 8162–8170. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.S.; Jaenicke, S.; Klähn, M. How the spontaneous insertion of amphiphilic imidazolium-based cations changes biological membranes: A molecular simulation study. Phys. Chem. Chem. Phys. 2015, 17, 29171–29183. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 218–225. [Google Scholar]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilibria 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Hansen, S.H.; Nordholm, L. N-alkylation of tertiary aliphatic amines by chloroform, dichloromethane and 1,2-dichloroethane. J. Chromatogr. A 1981, 204, 97–101. [Google Scholar] [CrossRef]
- Alvarez, V.H.; Dosil, N.; Gonzáles-Cabaleiro, R.; Mattedi, S.; Martin-Pastor, M.; Iglesias, M.; Navaza, J.M. Brønsted ionic liquids for sustainable process: Synthesis and physical properties. J. Chem. Eng. Data 2010, 55, 625–632. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Campusano, R.A.; Rojas, R.E. Glass transition temperature of ionic liquids using molecular descriptors and artificial neural networks. Comptes Rendus Chim. 2017, 20, 573–584. [Google Scholar] [CrossRef]
- Bae, J.H.; Sun, H.J.; Park, A.; Kim, D.; Yeon, J.; Kang, S.K.; Lee, E.H. Donepezil Multicomponent Systems: Donepezil–Water–Maleic Acid Crystals and More. Cryst. Growth Des. 2020, 20, 2283–2293. [Google Scholar] [CrossRef]
- Dasht Bozorg, B.; Banga, A.K. Effect of Different Pressure-Sensitive Adhesives on Performance Parameters of Matrix-Type Transdermal Delivery Systems. Pharmaceutics 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valia, K.H. Ramaraju vs. Inventors. Core Tech Solutions, Assignee. Transdermal Methods and Systems for Treating Alzheimer’s Disease. U.S. Patent US 20080044461A1, 21 February 2008. [Google Scholar]
- Madan, J.R.; Argade, N.S.; Dua, K. Formulation and evaluation of transdermal patches of donepezil. Recent Pat. Drug Deliv. Formul. 2015, 9, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, H.Y.; Lim, H.S.; Lee, S.H.; Jeon, H.S.; Hong, D.; Kim, S.S.; Choi, Y.K.; Bae, K.S. Single dose pharmacokinetics of the novel transdermal donepezil patch in healthy volunteers. Drug Des. Devel. Ther. 2015, 9, 1419–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| List of Dicarboxylic Acid Co-Former Candidates | ||
|---|---|---|
| adipic acid |  | 146.14 g/mol |
| aspartic acid |  | 133.11 g/mol |
| azelaic acid |  | 188.22 g/mol |
| fumaric acid |  | 116.07 g/mol |
| glutamic acid |  | 147.13 g/mol |
| glutaric acid |  | 132.12 g/mol |
| isophthalic acid |  | 166.14 g/mol |
| itaconic acid |  | 130.099 g/mol |
| maleic acid |  | 116.1 g/mol |
| malic acid |  | 134.0874 g/mol |
| phthalic acid |  | 166.14 g/mol |
| pimelic acid |  | 160.17 g/mol |
| saccharic acid |  | 210.1388 g/mol |
| sebacic acid |  | 202.25 g/mol |
| suberic acid |  | 174.2 g/mol |
| succinic acid |  | 118.09 g/mol |
| terephthalic acid |  | 166.13 g/mol |
| tartaric acid |  | 150.087 g/mol |
| Compound | Solubility in Water (mg/mL) | Solubility in 0.01 M PBS pH 7.4 (mg/mL) |
|---|---|---|
| DPZ free base | 0.007 ± 0.002 | 0.018 ± 0.001 |
| DPZ—adipic acid IL | 1.554 ± 0.235 | 1.561 ± 0.134 |
| DPZ—azelaic acid IL | 0.264 ± 0.046 | 1.308 ± 0.234 |
| DPZ—glutaric acid IL | 0.977 ± 0.105 | 1.250 ± 0.024 |
| DPZ—itaconic acid IL | 1.015 ± 0.028 | 1.915 ± 0.164 |
| DPZ—maleic acid IL | 0.677 ± 0.147 | 1.316 ± 0.129 |
| DPZ—malic acid IL | 1.892 ± 0.092 | 2.116 ± 0.055 |
| DPZ—phthalic acid IL | 0.313 ± 0.141 | 0.628 ± 0.106 |
| DPZ—pimelic acid IL | 0.660 ± 0.134 | 1.405 ± 0.227 |
| DPZ—sebacic acid IL | 0.287 ± 0.122 | 0.916 ± 0.058 |
| DPZ—suberic acid IL | 0.519 ± 0.136 | 1.686 ± 0.060 |
| DPZ—succinic acid IL | 1.283 ± 0.055 | 1.892 ± 0.243 |
| DPZ—tartaric acid IL | 1.407 ± 0.400 | 1.521 ± 0.067 |
| DPZ—alpha ketoglutaric IL | 1.894 ± 0.043 | 1.908 ± 0.226 |
| DURO-TAK® | Chemical Composition | Functional Groups | Cross-Linker | Solvent Composition | Tack | Solid Content | Viscosity |
|---|---|---|---|---|---|---|---|
| (oz/in2) | (%) | (mPa.s) | |||||
| 87-2074 | acrylate | –COOH–OH | present | 2-propanol, ethyl acetate, toluene | 20 | 29.5 | 1500 |
| 87-2051 | acrylate-vinyl acetate | –COOH | not present | ethyl acetate, N-heptane, vinyl acetate, cyclohexane | 80 | 51.5 | 4000 |
| 87-2196 | acrylate-vinyl acetate | –COOH | present | 2-propanol, ethyl acetate, toluene, vinyl acetate | 20 | 45 | 2100 |
| DPZ | DURO-TAK® 87-2051 | Adipic Acid | Azelaic Acid | PEG 400 | Tween 80 | Ethanol |
|---|---|---|---|---|---|---|
| g (%) | g (%) | g (%) | g (%) | g (%) | g (%) | (mL) |
| 0.4 (20) | 2.8 (72.5) | 0.15 (7.5) | 0.5 | |||
| 0.4 (20) | 2.68 (62.5) | 0.15 (7.5) | 0.2 (10) | 0.5 | ||
| 0.4 (20) | 2.796 (72) | 0.15 (7.5) | 0.01 (0.5) | 0.5 | ||
| 0.4 (20) | 2.8 (72.5) | 0.15 (7.5) | 0.5 | |||
| 0.4 (20) | 2.68 (62.5) | 0.15 (7.5) | 0.2 (10) | 0.5 | ||
| 0.4 (20) | 2.796 (72) | 0.15 (7.5) | 0.01 (0.5) | 0.5 |
| DPZ | DURO-TAK® 87-2074 | Adipic Acid | Azelaic Acid | PEG 400 | Tween 80 | Ethanol |
|---|---|---|---|---|---|---|
| g (%) | g (%) | g (%) | g (%) | g (%) | g (%) | (mL) |
| 0.4 (20) | 4.89 (72.5) | 0.15 (7.5) | 0.5 | |||
| 0.4 (20) | 4.68 (62.5) | 0.15 (7.5) | 0.2 (10) | 0.5 | ||
| 0.4 (20) | 4.88 (72) | 0.15 (7.5) | 0.01 (0.5) | 0.5 | ||
| 0.4 (20) | 4.89 (72.5) | 0.15 (7.5) | 0.5 | |||
| 0.4 (20) | 4.68 (62.5) | 0.15 (7.5) | 0.2 (10) | 0.5 | ||
| 0.4 (20) | 4.89 (72) | 0.15 (7.5) | 0.01 (0.5) | 0.5 |
| DPZ | DURO-TAK® 87-2196 | Adipic Acid | Azelaic Acid | PEG 400 | Tween 80 | Ethanol |
|---|---|---|---|---|---|---|
| g (%) | g (%) | g (%) | g (%) | g (%) | g (%) | (mL) |
| 0.4 (20) | 3.21 (72.5) | 0.15 (7.5) | 0.5 | |||
| 0.4 (20) | 3.07 (62.5) | 0.15 (7.5) | 0.2 (10) | 0.5 | ||
| 0.4 (20) | 3.2 (72) | 0.15 (7.5) | 0.01 (0.5) | 0.5 | ||
| 0.4 (20) | 3.21 (72.5) | 0.15 (7.5) | 0.5 | |||
| 0.4 (20) | 3.07 (62.5) | 0.15 (7.5) | 0.2 (10) | 0.5 | ||
| 0.4 (20) | 3.2 (72) | 0.15 (7.5) | 0.01 (0.5) | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh, L.; Lee, S.; Abuzar, S.M.; Park, H.; Hwang, S.-J. Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches. Pharmaceutics 2022, 14, 205. https://doi.org/10.3390/pharmaceutics14010205
Dinh L, Lee S, Abuzar SM, Park H, Hwang S-J. Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches. Pharmaceutics. 2022; 14(1):205. https://doi.org/10.3390/pharmaceutics14010205
Chicago/Turabian StyleDinh, Linh, Soohun Lee, Sharif Md Abuzar, Heejun Park, and Sung-Joo Hwang. 2022. "Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches" Pharmaceutics 14, no. 1: 205. https://doi.org/10.3390/pharmaceutics14010205