Enhanced Antimicrobial Action of Chlorhexidine Loaded in Shellac Nanoparticles with Cationic Surface Functionality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
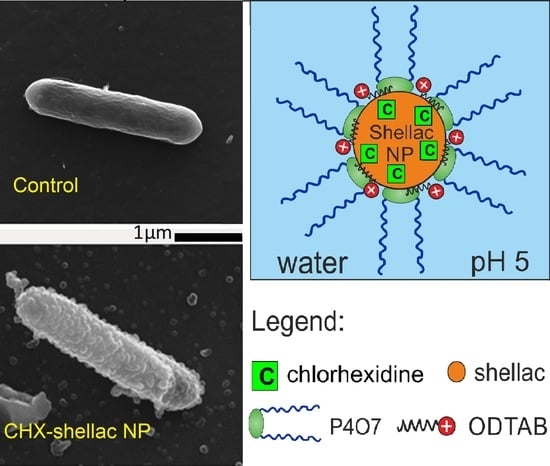
2.2. Preparation of CHX-Loaded Shellac NPs with Dual-Surface Functionality
2.3. CHX Encapsulation Efficiency, Drug Loading Content and Release Kinetics Measurements
2.4. Testing the Antimicrobial Action of CHX Formulations on E. coli
2.5. Testing the Antialgal Action of CHX Formulations on C. reinhardtii
2.6. Testing the Antimicrobial Action of CHX Formulations on S. cerevisiae
3. Results and Discussion
3.1. CHX Encapsulation and Characterization of CHX Loaded within Shellac NPs
3.2. FTIR and UV-Vis Spectra of Shellac NPs, Free CHX and CHX-Loaded Shellac NPs
3.3. CHX Encapsulation Efficiency and CHX Release Studies
3.4. Antimicrobial Activity of Free CHX and CHX-Loaded Shellac NPs on E. coli
3.5. Anti-Yeast Activity of Free CHX and CHX-Loaded Shellac NPs on S. Cerevisiae
3.6. Antialgal Activity of Free CHX and CHX-Loaded Shellac NPs on C. reinhardtii
3.7. Antibacterial Activity of ODTAB-Coated, CHX-Loaded Shellac NPs on E. coli
3.8. Anti-Yeast Activity of ODTAB-Coated, CHX-Loaded Shellac NPs on S. cerevisiae
3.9. Antialgal Activity of ODTAB-Coated, CHX-Loaded Shellac NPs on C. reinhardtii
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jenkins, S.; Addy, M.; Newcombe, R. Evaluation of a mouthrinse containing chlorhexidine and fluoride as an adjunct to oral hygiene. J. Clin. Periodontol. 1993, 20, 20–25. [Google Scholar] [CrossRef]
- Lang, N.P.; Hase, J.C.; Grassi, M.; Hammerle, C.H.; Weigel, C.; Kelty, E.; Frutig, F. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis. 1998, 4, 105–113. [Google Scholar] [CrossRef]
- Vianna, M.E.; Gomes, B.P.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.; de Souza-Filho, F.J. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 79–84. [Google Scholar] [CrossRef]
- Lboutounne, H.; Chaulet, J.-F.; Ploton, C.; Falsonm, F.; Pirot, F. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly(epsilon-caprolactone) nanocapsule encapsulated form and as a digluconate. J. Control. Release 2002, 82, 319–334. [Google Scholar] [CrossRef]
- Yue, I.C.; Poff, J.; Cortés, M.A.E.; Sinisterra, R.D.; Faris, C.B.; Hildgen, P.; Langer, R.; Shastri, V.P. A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomaterials 2004, 25, 3743–3750. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Leung, K.C.; Wong, C.H.; Lee, S.F.; Li, X.; Leung, P.C.; Lau, C.B.; Wat, E.; Jin, L. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS ONE 2014, 9, e103234. [Google Scholar] [CrossRef] [Green Version]
- Al-Awady, M.J.; Fauchet, A.; Greenway, G.M.; Paunov, V.N. Enhanced antimicrobial effect of berberine in nanogel carriers with cationic surface functionality. J. Mater. Chem. B 2017, 5, 7885–7897. [Google Scholar] [CrossRef] [PubMed]
- Al-Awady, M.J.; Weldrick, P.J.; Hardman, M.J.; Greenway, G.M.; Paunov, V.N. Amplified antimicrobial action of chlorhexidine encapsulated in PDAC-functionalized acrylate copolymer nanogel carriers. Mater. Chem. Front. 2018, 2, 2032–2044. [Google Scholar] [CrossRef]
- Al-Obaidy, S.S.M.; Greenway, G.M.; Paunov, V.N. Dual-functionalised shellac nanocarriers give a super-boost of the antimicrobial action of berberine. Nanoscale Adv. 2019, 1, 858–872. [Google Scholar] [CrossRef] [Green Version]
- Al-Obaidy, S.S.M.; Halbus, A.F.; Greenway, G.M.; Paunov, V.N. Boosting the antimicrobial action of vancomycin formulated in shellac nanoparticles of dual-surface functionality. J. Mater. Chem. B 2019, 7, 3119–3133. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Iveson, S.; Hardman, M.J.; Paunov, V.N. Breathing New Life into Old Antibiotics: Overcoming Antibacterial Resistance by Antibiotic-Loaded Nanogel Carriers with Cationic Surface Functionality. Nanoscale 2019, 11, 10472–10485. [Google Scholar] [CrossRef]
- Weldrick, P.J.; San, S.; Paunov, V.N. Advanced Alcalase-Coated Clindamycin-Loaded Carbopol Nanogels for Removal of Persistent Bacterial Biofilms. ACS Appl. Nano Mater. 2021, 4, 1187–1201. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and characterization of biodegradable lignin nanoparticles with tunable surface properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Controlling the antimicrobial action of surface modified magnesium hydroxide nanoparticles. Biomimetics 2019, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Surface-Modified Zinc Oxide Nanoparticles for Antialgal and Antiyeast Applications. ACS Appl. Nano Mater. 2020, 3, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Henry, P.; Halbus, A.F.; Athab, Z.H.; Paunov, V.N. Enhanced antimould action of surface modified copper oxide nanoparticles with phenylboronic acid surface functionality. Biomimetics 2021, 6, 19. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Self-grafting copper oxide nanoparticles show a strong enhancement of their anti-algal and anti-yeast action. Nanoscale Adv. 2019, 1, 2323–2336. [Google Scholar] [CrossRef] [Green Version]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Strongly Enhanced Antibacterial Action of Copper Oxide Nanoparticles with Boronic Acid Surface Functionality. ACS Appl. Mater. Interfaces 2019, 11, 12232–12243. [Google Scholar] [CrossRef] [PubMed]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. “Ghost” Silica Nanoparticles of “host”-Inherited Antibacterial Action. ACS Appl. Mater. Interfaces 2019, 11, 38519–38530. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Colloid particle formulations for antimicrobial applications. Adv. Colloid Interfaces Sci. 2017, 249, 134–148. [Google Scholar] [CrossRef]
- Al-Awady, M.J.; Greenway, G.M.; Paunov, V.N. Nanotoxicity of polyelectrolyte-functionalized titania nanoparticles towards microalgae and yeast: Role of the particle concentration, size and surface charge. RSC Adv. 2015, 5, 37044–37059. [Google Scholar] [CrossRef] [Green Version]
- Hagenmaier, R.D.; Shaw, P.E. Permeability of shellac coatings to gases and water vapor. J. Agric. Food Chem. 1991, 39, 825–829. [Google Scholar] [CrossRef]
- Hamad, S.A.; Stoyanov, S.D.; Paunov, V.N. Triggered cell release from shellac–cell composite microcapsules. Soft Matter 2012, 8, 5069–5077. [Google Scholar] [CrossRef]
- Hamad, S.A.; Amro, K.F.D.; Stoyanov, S.D.; Paunov, V.N. Sporopollenin microcapsules for microencapsulation of living cells. MRS Proc. 2012, 1499. [Google Scholar] [CrossRef]
- Hamad, S.A.; Stoyanov, S.D.; Paunov, V.N. Triggered release kinetics of living cells from composite microcapsules. Phys. Chem. Chem. Phys. 2013, 15, 2337–2344. [Google Scholar] [CrossRef]
- Leick, S.; Kott, M.; Degen, P.; Henning, S.; Pasler, T.; Suter, D.; Rehage, H. Mechanical properties of liquid-filled shellac composite capsules. Phys. Chem. Chem. Phys. 2011, 13, 2765–2773. [Google Scholar] [CrossRef]
- Farag, Y.; Leopold, C.S. Investigation of drug release from pellets coated with different shellac types. Drug Dev. Ind. Pharm. 2011, 37, 193–200. [Google Scholar] [CrossRef]
- Limmatvapirat, S.; Limmatvapirat, C.; Luangtana-Anan, M.; Nunthanid, J.; Oguchi, T.; Tozuka, Y.; Yamamoto, K.; Puttipipatkhachorn, S. Modification of physicochemical and mechanical properties of shellac by partial hydrolysis. Int. J. Pharm. 2004, 278, 41–49. [Google Scholar] [CrossRef]
- Patel, A.R.; Dorst, E.; Seijen ten Hoorn, J.; Velikov, K.P. Fabrication and characterization of emulsions with pH responsive switchable behavior. Soft Matter 2013, 9, 6747–6751. [Google Scholar] [CrossRef] [Green Version]
- Bellan, L.M.; Pearsall, M.; Cropek, D.M.; Langer, R. A 3D interconnected microchannel network formed in gelatin by sacrificial shellac microfibers. Adv. Mater. 2012, 24, 5187–5191. [Google Scholar] [CrossRef] [Green Version]
- Kraisit, P.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Luangtanaanan, M. Nanoparticle formation by using shellac and chitosan for a protein delivery system. Pharm. Dev. Technol. 2013, 18, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Heussen, P.; Hazekamp, J.; Velikov, K.P. Stabilisation and controlled release of silibinin from pH responsive shellac colloidal particles. Soft Matter 2011, 7, 8549–8555. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly(ethylene oxide) poly(propylene oxide) poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Super-Enhanced Removal of Fungal Biofilms by Protease-Functionalized Amphotericin B Nanocarriers. Adv. NanoBiomed Res. 2021, 1, 2000027. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. ACS Appl. Mater. Interfaces 2019, 11, 43902–43919. [Google Scholar] [CrossRef] [PubMed]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Smart active antibiotic nanocarriers with protease surface functionality can overcome biofilms of resistant bacteria. Mater. Chem. Front. 2021, 5, 961–972. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces: With Applications to Colloidal and Biological Systems, 2nd ed.; Academic Press: London, UK, 1992. [Google Scholar]
- Cold Spring Harbor Protocols. Available online: http://cshprotocols.cshlp.org/content/2009/9/pdb.rec11945.full?text_only=true (accessed on 1 September 2021).
- Kovtun, A.; Kozlova, D.; Ganesan, K.; Biewald, C.; Seipold, N.; Gaengler, P.; Arnold, W.H.; Epple, M. Chlorhexidine-loaded calcium phosphatenanoparticles for dental maintenance treatment: Combination of mineralising and antibacterial effects. RSC Adv. 2012, 2, 870–875. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123870186. [Google Scholar]
- Cai, X.; Han, B.; Liu, Y.; Tian, F.; Liang, F.; Wang, X. Chlorhexidine-Loaded Amorphous Calcium Phosphate Nanoparticles for Inhibiting Degradation and Inducing Mineralization of Type i Collagen. ACS Appl. Mater. Interfaces 2017, 9, 12949–12958. [Google Scholar] [CrossRef] [PubMed]
- Lboutounne, H.; Faivre, V.; Falson, F.; Pirot, F. Characterization of Transport of Chlorhexidine-Loaded Nanocapsules through Hairless and Wistar Rat Skin. J. Control. Release 2004, 17, 176–182. [Google Scholar] [CrossRef]
- Li, X.; Wong, C.-H.; Ng, T.-W.; Zhang, C.-F.; Leung, K.C.-F.; Jin, L. The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. Int. J. Nanomed. 2016, 11, 2471–2480. [Google Scholar] [CrossRef] [Green Version]
- Barreras, U.S.; Méndez, F.T.; Martínez, R.E.M.; Valencia, C.S.; Rodríguez, P.R.M.; Rodríguez, J.P.L. Chitosan nanoparticles enhance the antibacterial activity of chlorhexidine in collagen membranes used for periapical guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Desloges, I.; Sillard, C.; Bras, J. Controlled release and long-term antibacterial activity of chlorhexidine digluconate through the nanoporous network of microfibrillated cellulose. Cellulose 2014, 21, 4429–4442. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Obaidy, S.S.M.; Greenway, G.M.; Paunov, V.N. Enhanced Antimicrobial Action of Chlorhexidine Loaded in Shellac Nanoparticles with Cationic Surface Functionality. Pharmaceutics 2021, 13, 1389. https://doi.org/10.3390/pharmaceutics13091389
Al-Obaidy SSM, Greenway GM, Paunov VN. Enhanced Antimicrobial Action of Chlorhexidine Loaded in Shellac Nanoparticles with Cationic Surface Functionality. Pharmaceutics. 2021; 13(9):1389. https://doi.org/10.3390/pharmaceutics13091389
Chicago/Turabian StyleAl-Obaidy, Saba S. M., Gillian M. Greenway, and Vesselin N. Paunov. 2021. "Enhanced Antimicrobial Action of Chlorhexidine Loaded in Shellac Nanoparticles with Cationic Surface Functionality" Pharmaceutics 13, no. 9: 1389. https://doi.org/10.3390/pharmaceutics13091389