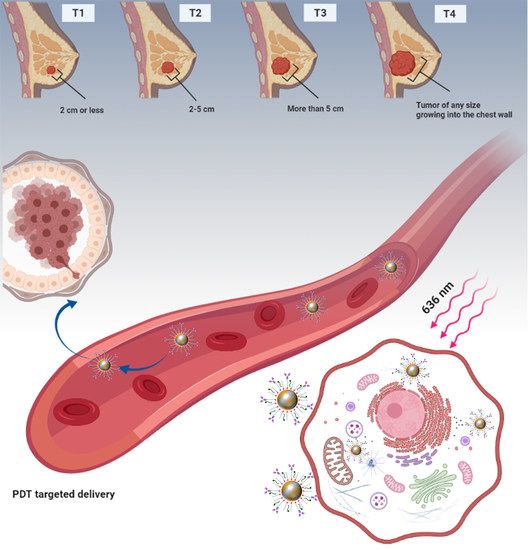
Targeted Photodynamic Therapy Using Alloyed Nanoparticle-Conjugated 5-Aminolevulinic Acid for Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bimetallic Alloyed Au–Ag NPs
2.3. Functionalization of the Au–Ag NPs with PEG, 5-ALA, and Ab
2.4. Characterization of NCs
2.5. Cell Culture and Preparation of Cell Culture Plates
2.6. PDT Laser Parameters and PS Addition
2.7. In Vitro Subcellular Localization of the PS and NCs
2.8. In Vitro Photodynamic Therapy of NCs
2.8.1. Morphology
2.8.2. ATP Cell Viability Assay
2.8.3. Flow Cytometry for Apoptosis and Necrosis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of the NCs
3.2. Molecular Characterizations of the NCs
3.2.1. UV–Visible Spectroscopy
3.2.2. Morphological and Energy Dispersive Spectroscopy (EDS) Analysis
3.2.3. Dynamic Light Scattering (DLS) and Zeta Potential (ZP)
3.2.4. FT-IR Analysis
3.2.5. Photostability of 5-ALA/Au–Ag-PEG Ab NCs
3.3. In Vitro Subcellular Uptake of the NCs
3.4. In Vitro Photodynamic Therapy Studies
3.4.1. Morphological Assessment
3.4.2. ATP Cell Viability Assay
3.4.3. Flow Cytometry Annexin V-FITC/PI Cell Death Pathway Detection Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP binding cassette |
| Abs | Antibodies |
| 5-ALA | 5-aminolevulinic acid |
| ATP | Adenosine triphosphate |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s modified eagle’s medium |
| EDS | Energy dispersive spectroscopy |
| EPR | Enhanced permeability and retention |
| FBS | Fetal bovine serum |
| FECH | Ferrochelatase |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| LSPR | Localized surface plasmon resonance |
| NC | Nanoconjugate |
| NPs | Nanoparticles |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity indexes |
| PDT | Photodynamic therapy |
| PEPT | Peptide transporter |
| PpIX | Protoporphyrin IX |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| SLC | Solute carrier |
| TPDT | Targeted photodynamic therapy |
| ZP | Zeta potential |
References
- Crescenzi, E.; Varriale, L.; Iovino, M.; Chiaviello, A.; Veneziani, B.M.; Palumbo, G. Photodynamic therapy with indocyanine green complements and enhances low-dose cisplatin cytotoxicity in MCF-7 breast cancer cells. Mol. Cancer Ther. 2004, 3, 537–544. [Google Scholar]
- Banerjee, S.; MacRobert, A.; Mosse, C.; Periera, B.; Bown, S.; Keshtgar, M. Photodynamic therapy: Inception to application in breast cancer. Breast 2017, 31, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Wu, J.; Han, H.; Jin, Q.; Li, Z.; Li, H.; Ji, J. Design and proof of programmed 5-aminolevulinic acid prodrug nanocarriers for targeted photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 14596–14605. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Sun, X.; Cheng, J.; Xu, H.; Dan, J.; Shen, J.; Zhou, Q.; Zhang, Y.; Meng, L.; Cao, W. Apoptosis of THP-1 macrophages induced by protoporphyrin IX-mediated sonodynamic therapy. Int. J. Nanomed. 2013, 8, 2239. [Google Scholar]
- Reinert, M.; Piffaretti, D.; Wilzbach, M.; Hauger, C.; Guckler, R.; Marchi, F.; D’angelo, M.L. Quantitative modulation of PpIX fluorescence and improved glioma visualization. Front. Surg. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, J.; Pottier, R.; Pross, D. Photodynamic therapy with endogenous protoporphyrin: IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B Biol. 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Ohgari, Y.; Nakayasu, Y.; Kitajima, S.; Sawamoto, M.; Mori, H.; Shimokawa, O.; Matsui, H.; Taketani, S. Mechanisms involved in δ-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: Relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochem. Pharmacol. 2005, 71, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Landes, R.; Illanes, A.; Goeppner, D.; Gollnick, H.; Friebe, M. A study of concentration changes of Protoporphyrin IX and Coproporphyrin III in mixed samples mimicking conditions inside cancer cells for Photodynamic Therapy. PLoS ONE 2018, 13, e0202349. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-i.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.; Liu, X.; Jiang, S.; Xiong, L. Desferrioxamine shows different potentials for enhancing 5-aminolaevulinic acid-based photodynamic therapy in several cutaneous cell lines. Lasers Med. Sci. 2010, 25, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, L.; Hu, S.; Liu, Y.; Ren, Y.; Zhang, X. Förster resonance energy transfer properties of a new type of near-infrared excitation PDT photosensitizer: CuInS 2/ZnS quantum dots-5-aminolevulinic acid conjugates. RSC Adv. 2016, 6, 55568–55576. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Kalscheuer, S.M.; Panyam, J. Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities. Adv. Drug Deliv. Rev. 2013, 65, 1731–1747. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-H.; Nam, K.C.; Cho, G.; Jung, J.-S.; Park, B.J. Enhanced photodynamic anticancer activities of multifunctional magnetic nanoparticles (Fe3O4) conjugated with chlorin e6 and folic acid in prostate and breast cancer cells. Nanomaterials 2018, 8, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, J.D.; Belekov, E.; Er, A.O.; Smith, M.E. Anti-cancer photodynamic therapy properties of sulphur-doped graphene quantum dot and methylene blue preparations in MCF-7 breast cancer cell culture. Photochem. Photobiol. 2019, 95, 1473–1481. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Liao, M.-L.; Hong, G.-C.; Chang, W.-W.; Chu, C.-C. Near-infrared-triggered photodynamic therapy toward breast cancer cells using dendrimer-functionalized upconversion nanoparticles. Nanomaterials 2017, 7, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Ge, Y.; Sun, Q.; Pan, W.; Wan, X.; Li, N.; Tang, B. A pre-protective strategy for precise tumor targeting and efficient photodynamic therapy with a switchable DNA/upconversion nanocomposite. Chem. Sci. 2018, 9, 3563–3569. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Xia, Y.; Xing, J.; Li, Z.; Zhen, J.; Jin, Y.; Tian, Y.; Liu, C.; Jiang, Z.; Li, J. Y 1-receptor–ligand-functionalized ultrasmall upconversion nanoparticles for tumor-targeted trimodality imaging and photodynamic therapy with low toxicity. Nanoscale 2018, 10, 17038–17052. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-García, G.; Panikar, S.S.; López-Luke, T.; Piazza, V.; Honorato-Colin, M.A.; Camacho-Villegas, T.; Hernández-Gutiérrez, R.; De la Rosa, E. An immunoconjugated up-conversion nanocomplex for selective imaging and photodynamic therapy against HER2-positive breast cancer. Nanoscale 2018, 10, 10154–10165. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, Y.; Zuo, J.; Tu, L.; Que, I.; Chang, Y.; Cruz, L.J.; Chan, A.; Zhang, H. Assembly of upconversion nanophotosensitizer in vivo to achieve scatheless real-time imaging and selective photodynamic therapy. Biomaterials 2019, 201, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.H.; Nam, J.M. Plasmonic photothermal nanoparticles for biomedical applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.-C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Sheny, D.; Mathew, J.; Philip, D. Phytosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 254–262. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Driskell, J.D. Quantifying bound and active antibodies conjugated to gold nanoparticles: A comprehensive and robust approach to evaluate immobilization chemistry. ACS Omega 2018, 3, 8253–8259. [Google Scholar] [CrossRef]
- Yokota, S. Preparation of colloidal gold particles and conjugation to protein A, IgG, F (ab’) 2, and streptavidin. In Immunoelectron Microscopy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 109–119. [Google Scholar]
- Sokolov, K.; Follen, M.; Aaron, J.; Pavlova, I.; Malpica, A.; Lotan, R.; Richards-Kortum, R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003, 63, 1999–2004. [Google Scholar] [PubMed]
- Ishikawa, T.; Kajimoto, Y.; Inoue, Y.; Ikegami, Y.; Kuroiwa, T. Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor. Adv. Cancer Res. 2015, 125, 197–216. [Google Scholar] [PubMed]
- Deng, F.; Sjöstedt, N.; Kidron, H. The effect of albumin on MRP2 and BCRP in the vesicular transport assay. PLoS ONE 2016, 11, e0163886. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Jajoo, A.; Dube, A. 5-Aminolevulinic acid-induced protoporphyrin-IX accumulation and associated phototoxicity in macrophages and oral cancer cell lines. J. Photochem. Photobiol. B Biol. 2007, 88, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, P.; Wedmgandt, H.; Baumgartner, R.; Kriegmair, M.; Hofstädter, F.; Knüchel, R. Cellular fluorescence of the endogenous photosensitizer protoporphyrin IX following exposure to 5-aminolevulinic acid. Photochem. Photobiol. 1995, 62, 887–895. [Google Scholar] [CrossRef]
- Liu, W.; Baer, M.R.; Bowman, M.J.; Pera, P.; Zheng, X.; Morgan, J.; Pandey, R.A.; Oseroff, A.R. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin. Cancer Res. 2007, 13, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, R.; Hagiya, Y.; Tamura, A.; Li, S.; Saito, H.; Tokushima, D.; Ishikawa, T. Cellular phototoxicity evoked through the inhibition of human ABC transporter ABCG2 by cyclin-dependent kinase inhibitors in vitro. Pharm. Res. 2009, 26, 449–458. [Google Scholar] [CrossRef]
- Golding, J.; Wardhaugh, T.; Patrick, L.; Turner, M.; Phillips, J.; Bruce, J.; Kimani, S. Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br. J. Cancer 2013, 109, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupusoru, R.V.; Pricop, D.A.; Uritu, C.M.; Arvinte, A.; Coroaba, A.; Esanu, I.; Zaltariov, M.F.; Silion, M.; Stefanescu, C.; Pinteala, M. Effect of TAT-DOX-PEG irradiated gold nanoparticles conjugates on human osteosarcoma cells. Sci. Rep. 2020, 10, 6591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Gonçalves, K.; da Silva, M.N.; Sicchieri, L.B.; de Oliveira Silva, F.R.; de Matos, R.A.; Courrol, L.C. Aminolevulinic acid with gold nanoparticles: A novel theranostic agent for atherosclerosis. Analyst 2015, 140, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, S.; Sanjay, K. Conjugation study of 5-aminolevulinic acid with microbial synthesized gold nanoparticles to evaluate its effect on skin melanoma and epidermoid carcinoma cell lines using photodynamic cancer therapy. Gold Bull. 2018, 51, 11–19. [Google Scholar] [CrossRef]
- Chung, C.-W.; Chung, K.-D.; Jeong, Y.-I.; Kang, D.H. 5-aminolevulinic acid-incorporated nanoparticles of methoxy poly (ethylene glycol)-chitosan copolymer for photodynamic therapy. Int. J. Nanomed. 2013, 8, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegoke, O.; Morita, M.; Kato, T.; Ito, M.; Suzuki, T.; Park, E.Y. Localized surface plasmon resonance-mediated fluorescence signals in plasmonic nanoparticle-quantum dot hybrids for ultrasensitive Zika virus RNA detection via hairpin hybridization assays. Biosens. Bioelectron. 2017, 94, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, C.; Mei, J.; Yao, C.; Wang, S.; Wang, J.; Li, Z.; Zhang, Z. Effects of light irradiation upon photodynamic therapy based on 5-aminolevulinic acid–gold nanoparticle conjugates in K562 cells via singlet oxygen generation. Int. J. Nanomed. 2012, 7, 5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulvaney, P. Metal nanoparticles: Double layers, optical properties, and electrochemistry. In Nanoscale Materials in Chemistry; Klabunde, K.J., Ed.; Wiley: New York, NY, USA, 2001; pp. 121–167. [Google Scholar] [CrossRef]
- Kuipers, B.J.; Gruppen, H. Prediction of molar extinction coefficients of proteins and peptides using UV absorption of the constituent amino acids at 214 nm to enable quantitative reverse phase high-performance liquid chromatography–mass spectrometry analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, C.-X.; Chen, L.-G.; Yan, X.-P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017, 8, 14998. [Google Scholar] [CrossRef] [Green Version]
- Davatgaran Taghipour, Y.; Kharrazi, S.; Amini, S.M. Antibody conjugated gold nanoparticles for detection of small amounts of antigen based on surface plasmon resonance (SPR) spectra. Nanomed. Res. J. 2018, 3, 102–108. [Google Scholar]
- Yao, G.-Y.; Liu, Q.-L.; Zhao, Z.-Y. Studied localized surface plasmon resonance effects of Au nanoparticles on TiO2 by FDTD simulations. Catalysts 2018, 8, 236. [Google Scholar] [CrossRef]
- Koizumi, N.; Hanai, T. Dielectric properties of polyethylene glycols: Dielectric relaxation in solid state (special issue on polymer chemistry, I). Bull. Inst. Chem. Res. Kyoto Univ. 1964, 42, 115–127. [Google Scholar]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Khaing Oo, M.K.; Yang, X.; Du, H.; Wang, H. 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. Nanomedicine 2008, 3, 777–786. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Seong, J.S.; Park, S.N. Preparation of novel capsosome with liposomal core by layer-by-Layer self-assembly of sodium hyaluronate and chitosan. Colloids Surf. B 2016, 144, 99–107. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, K.; Vieira, D.P.; Levy, D.; Bydlowski, S.P.; Courrol, L.C. Uptake of silver, gold, and hybrids silver-iron, gold-iron and silver-gold aminolevulinic acid nanoparticles by MCF-7 breast cancer cells. Photodiagn. Photodyn. Ther. 2020, 32, 102080. [Google Scholar] [CrossRef]
- Shaker, M.N.; Ramadan, H.S.; Mohamed, M.M.; Roston, G.D. Enhanced photodynamic efficacy of PLGA-encapsulated 5-ALA nanoparticles in mice bearing Ehrlich ascites carcinoma. Appl. Nanosci. 2014, 4, 777–789. [Google Scholar] [CrossRef] [Green Version]
- Punjabi, A.; Wu, X.; Tokatli-Apollon, A.; El-Rifai, M.; Lee, H.; Zhang, Y.; Wang, C.; Liu, Z.; Chan, E.M.; Duan, C. Amplifying the red-emission of upconverting nanoparticles for biocompatible clinically used prodrug-induced photodynamic therapy. ACS Nano 2014, 8, 10621–10630. [Google Scholar] [CrossRef]
- Aghamiri, S.; Jafarpour, A.; Shoja, M. Effects of silver nanoparticles coated with anti-Her2 on irradiation efficiency of Skbr3 breast cancer cells. IET Nanobiotechnol. 2019, 13, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Juluri, B.K.; Zheng, Y.B.; Ahmed, D.; Jensen, L.; Huang, T.J. Effects of geometry and composition on charge-induced plasmonic shifts in gold nanoparticles. J. Phys. Chem. C 2008, 112, 7309–7317. [Google Scholar] [CrossRef]
- Hoener, B.S.; Zhang, H.; Heiderscheit, T.S.; Kirchner, S.R.; De Silva Indrasekara, A.S.; Baiyasi, R.; Cai, Y.; Nordlander, P.; Link, S.; Landes, C.F. Spectral response of plasmonic gold nanoparticles to capacitive charging: Morphology effects. J. Phys. Chem. Lett. 2017, 8, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Gadmar, Ø.B.; Moan, J.; Scheie, E.; Ma, L.-W.; Peng, Q. The stability of 5-aminolevulinic acid in solution. J. Photochem. Photobiol. B Biol. 2002, 67, 187–193. [Google Scholar] [CrossRef]
- Steluti, R.; De Rosa, F.S.; Collett, J.; Tedesco, A.C.; Bentley, M.V.L.B. Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. Eur. J. Pharm. Biopharm. 2005, 60, 439–444. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Qian, J.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Sequentially self-assembled polysaccharide-based nanocomplexes for combined chemotherapy and photodynamic therapy of breast cancer. Carbohydr. Polym. 2019, 203, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]







| NPs/NCs | Hydrodynamic Size (nm) DLS | Polydispersity Index DLS | Zeta Potential (mV) ELS |
|---|---|---|---|
| Anti-HER-2 Ab | - | - | −3.6 ± 1.2 |
| 5-ALA | - | - | −1.3 ± 0.7 |
| Citrate Au–Ag NPs | 33.8 ± 4.4 | 0.71 ± 0.08 | −21.0 ± 0.3 |
| Au–Ag-PEG | 154.0 ± 1.2 | 0.29 ± 0.01 | 7.5 ± 0.7 |
| 5-ALA/Au–Ag-PEG | 192.4 ± 3.0 | 0.26 ± 0.04 | 5.5 ± 0.8 |
| 5-ALA/Au–Ag-PEG-Ab | 185.6 ± 1.4 | 0.29 ± 0.02 | 5.8 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montaseri, H.; Kruger, C.A.; Abrahamse, H. Targeted Photodynamic Therapy Using Alloyed Nanoparticle-Conjugated 5-Aminolevulinic Acid for Breast Cancer. Pharmaceutics 2021, 13, 1375. https://doi.org/10.3390/pharmaceutics13091375
Montaseri H, Kruger CA, Abrahamse H. Targeted Photodynamic Therapy Using Alloyed Nanoparticle-Conjugated 5-Aminolevulinic Acid for Breast Cancer. Pharmaceutics. 2021; 13(9):1375. https://doi.org/10.3390/pharmaceutics13091375
Chicago/Turabian StyleMontaseri, Hanieh, Cherie Ann Kruger, and Heidi Abrahamse. 2021. "Targeted Photodynamic Therapy Using Alloyed Nanoparticle-Conjugated 5-Aminolevulinic Acid for Breast Cancer" Pharmaceutics 13, no. 9: 1375. https://doi.org/10.3390/pharmaceutics13091375