Thermosensitive Bioadhesive Hydrogels Based on Poly(N-isopropylacrilamide) and Poly(methyl vinyl ether-alt-maleic anhydride) for the Controlled Release of Metronidazole in the Vaginal Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
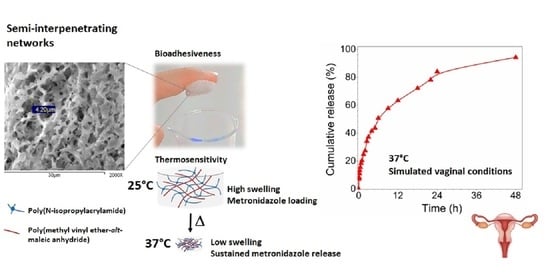
2.2. Preparation of s-IPN
2.3. Characterizations
2.4. Bioadhesion Analysis
2.5. Loading and Releasing Studies of MTZ
3. Results and Discussion
3.1. FTIR
3.2. SEM Analysis
3.3. TGA Analysis
3.4. Rheological Measurements
3.5. Swelling Kinetic Measurements
3.6. Bioadhesive Analysis
3.7. Studies of MTZ Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, T.M.D.; Nguyen, V.V.L.; Trinh, T.A.; Pham, N.S.; Lee, D.S.; Huynh, D.P. Sulfonamide functionalized amino acid-based pH- and temperature-sensitive biodegradable injectable hydrogels: Synthesis, physicochemical characterization and in vivo degradation kinetics. J. Appl. Polym. Sci. 2021, 138, 50488. [Google Scholar] [CrossRef]
- Xue, P.; Wang, L.; Xu, J.; Liu, J.; Pan, X.; Zhao, Y.; Xu, H. Temperature-sensitive hydrogel for rectal perfusion improved the therapeutic effect of Kangfuxin liquid on DSS-induced ulcerative colitis mice: The inflammation alleviation and the colonic mucosal barriers repair. Int. J. Pharm. 2020, 589, 119846. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Motokawa, R.; Morishita, K.; Koizumi, S.; Nakahira, T.; Annaka, M. Thermosensitive diblock copolymer of poly(N-isopropylacrylamide) and poly(ethylene glycol) in water: Polymer preparation and solution behavior. Macromolecules 2005, 38, 5748–5760. [Google Scholar] [CrossRef]
- Hay, D.N.T.; Rickert, P.G.; Seifert, S.; Firestone, M.A. Thermoresponsive Nanostructures by Self-Assembly of a Poly(N-isopropylacrylamide)-Lipid Conjugate. J. Am. Chem. Soc. 2004, 126, 2290–2291. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A.; Dubovik, A.S.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y. Temperature-sensitive chitosan-poly(N-isopropylacrylamide) interpenetrated networks with enhanced loading capacity and controlled release properties. J. Control. Release 2005, 102, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Stile, R.A.; Healy, K.E. Poly(N-isopropylacrylamide)-based semi-interpenetrating polymer networks for tissue engineering applications. 1. Effects of linear poly(acrylic acid) chains on phase behavior. Biomacromolecules 2002, 3, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Çaykara, T.; Kiper, S.; Demirel, G. Network parameters and volume phase transition behavior of poly(N-isopropylacrylamide) hydrogels. J. Appl. Polym. Sci. 2006, 101, 1756–1762. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.D.; de Freitas, O.; Lara, E.H.G. Semisolid Systems Containing Propolis for the Treatment of Periodontal Disease: In Vitro Release Kinetics, Syringeability, Rheological, Textural, and Mucoadhesive Properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Kaur, G. Innovations in bioadhesive vaginal drug delivery system. Expert Opin. Ther. Pat. 2012, 22, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Imperiale, J.C.; Vázquez-González, B.; Raskin, M.M.; Muñoz-Muñoz, F.; Burillo, G.; Cedillo, G.; Bucio, E. Mucoadhesive thermo-responsive chitosan-g-poly(N-isopropylacrylamide) polymeric micelles via a one-pot gamma-radiation-assisted pathway. Colloids Surf. B Biointerfaces 2015, 136, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Wiltsey, C.; Christiani, T.; Williams, J.; Scaramazza, J.; Van Sciver, C.V.; Toomer, K.; Sheehan, J.; Branda, A.; Nitzl, A.; England, E.; et al. Thermogelling bioadhesive scaffolds for intervertebral disk tissue engineering: Preliminary in vitro comparison of aldehyde-based versus alginate microparticle-mediated adhesion. Acta Biomater. 2015, 16, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemetsrud, T.; Kjøniksen, A.L.; Hiorth, M.; Jacobsen, J.; Smistad, G. Polymer coated liposomes for use in the oral cavity—A study of the in vitro toxicity, effect on cell permeability and interaction with mucin. J. Liposome Res. 2018, 28, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.P.; Joon, I.Y.; Li, H.; Dong, C.M.; Han, K. Buccal mucosal ulcer healing effect of rhEGF/eudispert hv hydrogel. Arch. Pharm. Res. 2003, 26, 659–665. [Google Scholar]
- Menard, J.P. Antibacterial treatment of bacterial vaginosis: Current and emerging therapies. Int. J. Womens Health 2011, 3, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef]
- Rajan, S.S.; Cavera, V.L.; Zhang, X.; Singh, Y.; Chikindas, M.L.; Sinko, P.J. Polyethylene glycol-based hydrogels for controlled release of the antimicrobial subtilosin for prophylaxis of bacterial vaginosis. Antimicrob. Agents Chemother. 2014, 58, 2747–2753. [Google Scholar] [CrossRef] [Green Version]
- Malli, S.; Bories, C.; Pradines, B.; Loiseau, P.M.; Ponchel, G.; Bouchemal, K. In situ forming pluronic® F127/chitosan hydrogel limits metronidazole transmucosal absorption. Eur. J. Pharm. Biopharm. 2017, 112, 143–147. [Google Scholar] [CrossRef]
- Giordani, B.; Abruzzo, A.; Musazzi, U.M.; Cilurzo, F.; Nicoletta, F.P.; Dalena, F.; Parolin, C.; Vitali, B.; Cerchiara, T.; Luppi, B.; et al. Freeze-Dried Matrices Based on Polyanion Polymers for Chlorhexidine Local Release in the Buccal and Vaginal Cavities. J. Pharm. Sci. 2019, 108, 2447–2457. [Google Scholar] [CrossRef]
- Chhabra, H.; Gupta, P.; Verma, P.J.; Jadhav, S.; Bellare, J.R. Gelatin–PMVE/MA composite scaffold promotes expansion of embryonic stem cells. Mater. Sci. Eng. C 2014, 37, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Ozer, F.; Mante, F.K. Fracture mechanics of dental adhesives supplemented with Polymethyl-vinyl-ether-co-maleic anhydride. J. Adhes. Sci. Technol. 2017, 31, 1116–1124. [Google Scholar] [CrossRef]
- Demir, Y.K.; Metin, A.Ü.; Şatıroğlu, B.; Solmaz, M.E.; Kayser, V.; Mäder, K. Poly (methyl vinyl ether-co-maleic acid)—Pectin based hydrogel-forming systems: Gel, film, and microneedles. Eur. J. Pharm. Biopharm. 2017, 117, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Mazi, H.; Gulpinar, A. Cu(II), Zn(II) and Mn(II) complexes of poly(methyl vinyl ether-alt-maleic anhydride). Synthesis, characterization and thermodynamic parameters. J. Chem. Sci. 2014, 126, 239–245. [Google Scholar] [CrossRef]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Ryu, I.S.; Liu, X.; Jin, Y.; Sun, J.; Lee, Y.J. Stoichiometric analysis of competing intermolecular hydrogen bonds using infrared spectroscopy. RSC Adv. 2018, 8, 23481–23488. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Morphological and mechanical properties of tannic acid/PAAm semi-IPN hydrogels for cell adhesion. Polym. Test. 2017, 61, 314–323. [Google Scholar] [CrossRef]
- Meena, L.K.; Raval, P.; Kedaria, D.; Vasita, R. Study of locust bean gum reinforced cyst-chitosan and oxidized dextran based semi-IPN cryogel dressing for hemostatic application. Bioact. Mater. 2018, 3, 370–384. [Google Scholar] [CrossRef]
- Apopei Loghin, D.F.; Biliuta, G.; Coseri, S.; Dragan, E.S. Preparation and characterization of oxidized starch/poly(N,N-dimethylaminoethyl methacrylate) semi-IPN cryogels and in vitro controlled release evaluation of indomethacin. Int. J. Biol. Macromol. 2017, 96, 589–599. [Google Scholar] [CrossRef]
- Wang, D.; Xia, Y.; Zhang, D.; Sun, X.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Hydrogen-Bonding Reinforced Injectable Hydrogels: Application As a Thermo-Triggered Drug Controlled-Release System. ACS Appl. Polym. Mater. 2020, 2, 1587–1596. [Google Scholar] [CrossRef]
- Chung, K.H.; Wu, C.S.; Malawer, E.G. Glass transition temperatures of poly(methyl vinyl ether-co-maleic anhydride) (PMVEMA) and poly(methyl vinyl ether-co-maleic acid) (PMVEMAC) and the kinetics of dehydration of PMVEMAC by thermal analysis. J. Appl. Polym. Sci. 1990, 41, 793–803. [Google Scholar] [CrossRef]
- Schild, H.G. Thermal decomposition of PNIPAAM: TGA-FTIR analysis. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2259–2262. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y. A novel pH/temperature-responsive hydrogel based on tremella polysaccharide and poly(N-isopropylacrylamide). Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124270. [Google Scholar] [CrossRef]
- Nishinari, K. Rheological and DSC study of sol-gel transition in aqueous dispersions of industrially important polymers and colloids. Colloid Polym. Sci. 1997, 275, 1093–1107. [Google Scholar] [CrossRef]
- Raj Singh, T.R.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Investigation of swelling and network parameters of poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels. Eur. Polym. J. 2009, 45, 1239–1249. [Google Scholar] [CrossRef]
- Zong, Y.; Wei, Y.; Morgan, S.E. Adsorption/desorption processes of ph-responsive copolymers on model dental surfaces via QCM and AFM analysis. In Polymers for Personal Care and Cosmetics; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1148, pp. 301–318. [Google Scholar]
- Lin, J.; Pozharski, E.; Wilson, M.A. Short carboxylic acid-carboxylate hydrogen bonds can have fully localized protons. Biochemistry 2017, 56, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Sachan, V.K.; Devi, A.; Katiyar, R.S.; Nagarale, R.K.; Bhattacharya, P.K. Proton transport properties of sulphanilic acid tethered poly(methyl vinyl ether-alt-maleic anhydride)-PVA blend membranes. Eur. Polym. J. 2014, 56, 45–58. [Google Scholar] [CrossRef]
- Arbós, P.; Campanero, M.A.; Arangoa, M.A.; Renedo, M.J.; Irache, J.M. Influence of the surface characteristics of PVM/MA nanoparticles on their bioadhesive properties. J. Control. Release 2003, 89, 19–30. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different Types of Gel Carriers as Metronidazole Delivery Systems to the Oral Mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef] [Green Version]
- Perioli, L.; Ambrogi, V.; Venezia, L.; Pagano, C.; Ricci, M.; Rossi, C. Chitosan and a modified chitosan as agents to improve performances of mucoadhesive vaginal gels. Colloids Surf. B Biointerfaces 2008, 66, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhuo, R. Crosslinked Quaternary Ammonium Cornstarch Matrix for Slow Release of Carboxylic Groups-containing Herbicides. Starch Stärke 2000, 52, 58–63. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Awad, G.A.S.; Mansour, S.; El-Shamy, A.E.H.A. Release mechanisms behind polysaccharides-based famotidine controlled release matrix tablets. AAPS PharmSciTech 2008, 9, 1230–1239. [Google Scholar] [CrossRef] [Green Version]
- Perinelli, D.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Code | NIPAAm/MBA (mL) | PMVE-alt-MA (mL) |
|---|---|---|
| PNIPAAm | 2 | 0 |
| s-IPN 1 | 1.60 | 0.40 |
| s-IPN 2 | 1.46 | 0.54 |
| s-IPN 3 | 1.34 | 0.66 |
| Composition in Water | g·L−1 |
|---|---|
| Sodium chloride | 3.51 |
| Potassium hydroxide | 1.40 |
| Calcium hydroxide | 0.22 |
| Bovine serum albumin | 0.02 |
| Lactic acid | 2.00 |
| Acetic acid | 1.00 |
| Glycerol | 0.16 |
| Urea | 0.40 |
| Glucose | 5.00 |
| Sample | Tmax (°C) |
|---|---|
| PNIPAAm | 410.29 |
| s-IPN 1 | 173.36, 395.77, 596.5 |
| s-IPN 2 | 168.35, 282.6, 419.63, 622.56 |
| s-IPN 3 | 173.59, 284.69, 415.24, 612.47 |
| PVME-MA | 170.31, 280.86, 444.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Figueroa, A.V.; Pérez-Martínez, C.J.; Encinas, J.C.; Burruel-Ibarra, S.; Silvas-García, M.I.; García Alegría, A.M.; del Castillo-Castro, T. Thermosensitive Bioadhesive Hydrogels Based on Poly(N-isopropylacrilamide) and Poly(methyl vinyl ether-alt-maleic anhydride) for the Controlled Release of Metronidazole in the Vaginal Environment. Pharmaceutics 2021, 13, 1284. https://doi.org/10.3390/pharmaceutics13081284
Torres-Figueroa AV, Pérez-Martínez CJ, Encinas JC, Burruel-Ibarra S, Silvas-García MI, García Alegría AM, del Castillo-Castro T. Thermosensitive Bioadhesive Hydrogels Based on Poly(N-isopropylacrilamide) and Poly(methyl vinyl ether-alt-maleic anhydride) for the Controlled Release of Metronidazole in the Vaginal Environment. Pharmaceutics. 2021; 13(8):1284. https://doi.org/10.3390/pharmaceutics13081284
Chicago/Turabian StyleTorres-Figueroa, Ana V., Cinthia J. Pérez-Martínez, J. Carmelo Encinas, Silvia Burruel-Ibarra, María I. Silvas-García, Alejandro M. García Alegría, and Teresa del Castillo-Castro. 2021. "Thermosensitive Bioadhesive Hydrogels Based on Poly(N-isopropylacrilamide) and Poly(methyl vinyl ether-alt-maleic anhydride) for the Controlled Release of Metronidazole in the Vaginal Environment" Pharmaceutics 13, no. 8: 1284. https://doi.org/10.3390/pharmaceutics13081284