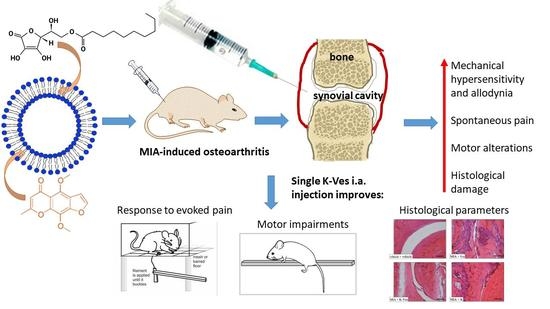
The Anti-Arthritic Efficacy of Khellin Loaded in Ascorbyl Decanoate Nanovesicles after an Intra-Articular Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanovesicles
2.3. Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS)
2.4. Morphological Characterization
2.5. HPLC-DAD Analyses
2.6. Encapsulation Efficiency and Recovery
2.7. Stability Studies in Simulated Synovial Fluid
2.8. Animals
2.9. MIA-Induced Osteoarthritis
2.10. Treatments
2.11. Paw Pressure Test
2.12. Von Frey Test
2.13. Incapacitance Test
2.14. Beam Balance Test
2.15. Histological Studies
2.16. Statistical Analysis
3. Results and Discussion
3.1. Formulation and Characterization of Nanovesicles
3.2. Khellin R% and EE%
3.3. Stability Studies of K-Ves in Simulated Synovial Fluid
3.4. Behavioral Evaluation
3.5. Histological Evaluation of Synovia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carr, A.J. Beyond disability: Measuring the social and personal consequences of osteoarthritis. Osteoarthr. Cartil. 1999, 7, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 2014, 95, 986.e1–995.e1. [Google Scholar] [CrossRef] [Green Version]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar] [PubMed]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef] [Green Version]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Larsen, C.; Østergaard, J.; Larsen, S.W.; Jensen, H.; Jacobsen, S.; Lindegaard, C.; Andersen, P.H. Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders. J. Pharm. Sci. 2008, 97, 4622–4654. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Kraus, V.; Setton, L. Progress in intra-articular therapy. Rheumatol. Nat. Rev. 2015, 10, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Mehtaa, S.; Hea, T.; Bajpayee, A.G. Recent advances in targeted drug delivery for treatment of osteoarthritis. Curr. Opinion Rheumatol. 2021, 33, 94–109. [Google Scholar] [CrossRef]
- Lee, J.K.; Jung, J.S.; Park, S.H.; Park, S.H.; Sim, Y.B.; Kim, S.M.; Ha, T.S.; Suh, H.W. Anti-inflammatory effect of visnagin in lipopolysaccharide-stimulated BV-2 microglial cells. Arch. Pharm. Res. 2010, 33, 1843–1850. [Google Scholar] [CrossRef]
- Risaliti, L.; Ambrosi, M.; Calamante, M.; Bergonzi, M.C.; Lo Nostro, P.; Bilia, A.R. Preparation and characterization of ascosome vesicles loaded with khellin. J. Pharm. Sci. 2020, 109, 3114–3124. [Google Scholar] [CrossRef]
- Capuzzi, G.; Lo Nostro, P.; Kulkarni, K.; Fernandez, J.E. Mixtures of stearoyl-6-O-ascorbic acid and α-tocopherol: A monolayer study at the gas/water interface. Langmuir 1996, 12, 3957–3963. [Google Scholar] [CrossRef]
- Vanti, G.; Bani, D.; Salvatici, M.C.; Bergonzi, M.C.; Bilia, A.R. Development and percutaneous permeation study of escinosomes, escin-based nanovesicles loaded with berberine chloride. Pharmaceutics 2019, 11, 682. [Google Scholar] [CrossRef] [Green Version]
- Vanti, G.; Coronnello, M.; Bani, D.; Mannini, A.; Bergonzi, M.C.; Bilia, A.R. Co-delivery of berberine chloride and tariquidar in nanoliposomes enhanced intracellular berberine chloride in a doxorubicin-resistant K562 cell line due to P-gp overexpression. Pharmaceutics 2021, 13, 306. [Google Scholar] [CrossRef]
- Vanti, G.; Tomou, E.M.; Stojković, D.; Ćirić, A.; Bilia, A.R.; Skaltsa, H. Nanovesicles loaded with Origanum onites and Satureja thymbra essential oils and their activity against food-borne pathogens and spoilage microorganisms. Molecules 2021, 26, 2124. [Google Scholar] [CrossRef]
- Vanti, G.; Wang, M.; Bergonzi, M.C.; Zhidong, L.; Bilia, A.R. Hydroxypropyl methylcellulose hydrogel of berberine chloride-loaded escinosomes: Dermal absorption and biocompatibility. Int. J. Biol. Macromol. 2020, 164, 232–241. [Google Scholar] [CrossRef]
- Bortel, E.L.; Charbonnier, B.; Heuberger, R. Development of a synthetic synovial fluid for tribological testing. Lubricants 2015, 3, 664–686. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low dose native type II collagen prevents pain in a rat osteoarthritis model. BMC Musculoskelet. Disord 2013, 14, 228. [Google Scholar] [CrossRef] [Green Version]
- Maresca, M.; Micheli, L.; Cinci, L.; Bilia, A.R.; Ghelardini, C.; Di Cesare Mannelli, L. Pain relieving and protective effects of Astragalus hydroalcoholic extract in rat arthritis models. J. Pharm. Pharmacol. 2017, 69, 1858–1870. [Google Scholar] [CrossRef] [Green Version]
- Micheli, L.; Ghelardini, C.; Lucarini, E.; Parisio, C.; Trallori, E.; Cinci, L.; Di Cesare Mannelli, L. Intra-articular mucilages: Behavioural and histological evaluations for a new model of articular pain. J. Pharm. Pharmacol. 2019, 71, 971–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leighton, G.E.; Rodriguez, R.E.; Hill, R.G.; Hughes, J. κ-Opioid agonist produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br. J. Pharmacol. 1988, 93, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Bird, M.F.; Cerlesi, M.C.; Brown, M.; Malfacini, D.; Vezzi, V.; Molinari, P.; Micheli, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Guerrini, R.; et al. Characterisation of the novel mixed mu-NOP peptide ligand dermorphin-N/OFQ (DeNo). PLoS ONE 2016, 11, e0156897. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, M.; Egashira, N.; Kawashiri, T.; Yano, T.; Ikesue, H.; Oishi, R. Oxaliplatin-induced neuropathy in the rat: Involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain 2009, 147, 165–174. [Google Scholar] [CrossRef]
- Baptista-de-Souza, D.; Di Cesare Mannelli, L.; Zanardelli, M.; Micheli, L.; Nunes-de-Souza, R.L.; Canto-de-Souza, A.; Ghelardini, C. Serotonergic modulation in neuropathy induced by oxaliplatin: Effect on the 5HT2C receptor. Eur. J. Pharmacol. 2014, 735, 141–149. [Google Scholar] [CrossRef]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Maresca, M.; Micheli, L.; Di Cesare Mannelli, L.; Tenci, B.; Innocenti, M.; Khatib, M.; Mulinacci, N.; Ghelardini, C. Acute effect of Capparis spinosa root extracts on rat articular pain. J. Ethnopharmacol. 2016, 193, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, J.; Lai, Q.; Rafols, J.A.; Luan, X.; Clark, J.; Diaz, F.G. Motor balance and coordination training enhances functional outcome in rat with transient middle cerebral artery occlusion. Neuroscience 2004, 123, 667–674. [Google Scholar] [CrossRef]
- Snekhalatha, U.; Anburajan, M.; Venkatraman, B.; Menaka, M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Comparison of thermography and histopathology. Z. Rheumatol. 2013, 72, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Bozdag, M.; Akgul, O.; Carta, F.; Guccione, C.; Bergonzi, M.C.; Bilia, A.R.; Cinci, L.; Lucarini, E.; Parisio, C.; et al. Pain relieving effect of-NSAIDs-CAIs hybrid molecules: Systemic and intra-articular treatments against rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 1923. [Google Scholar] [CrossRef]
- Combe, R.; Bramwell, S.; Field, M.J. The monosodium iodoacetate model of osteoarthritis: A model of chronic nociceptive pain in rats? Neurosci. Lett. 2004, 370, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Di Cesare Mannelli, L.; Mattoli, L.; Tamimi, S.; Flamini, E.; Garetto, S.; Lucci, J.; Giovagnoni, E.; Cinci, L.; D’Ambrosio, M.; et al. Intra-articular route for the system of molecules 14G1862 from Centella asiatica: Pain relieving and protective effects in a rat model of osteoarthritis. Nutrients. 2020, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The role of nanomedicine in the development of clinical natural drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A successful tool to increase solubility, stability and optimise bioefficacy of natural constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef] [PubMed]
- FDA. Liposome Drug Products; Chemistry Manufacturing and Controls; Human Pharmacokinetics and Bioavailability; Labeling Documentation; Guidance for Industry; April 2018 Pharmaceutical Quality/CMC; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2018.









| Ascorbyl Decanoate (mg/mL) | P90G (mg/mL) | PDI | Size (nm) | ζ-Potential (mV) |
|---|---|---|---|---|
| 3.2 | 13.8 | 0.38 ± 0.02 | 214.7 ± 1.5 | −36.2 ± 0.3 |
| 6.4 | 13.8 | 0.32 ± 0.02 | 172.8 ± 2.9 | −44.2 ± 0.1 |
| 9.6 | 13.8 | 0.26 ± 0.01 | 134.0 ± 0.7 | −44.5 ± 0.3 |
| 13.2 | 13.8 | 0.27 ± 0.01 | 128.7 ± 2.0 | −43.1 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanti, G.; Di Cesare Mannelli, L.; Micheli, L.; Cinci, L.; Grifoni, L.; Bergonzi, M.C.; Ghelardini, C.; Bilia, A.R. The Anti-Arthritic Efficacy of Khellin Loaded in Ascorbyl Decanoate Nanovesicles after an Intra-Articular Administration. Pharmaceutics 2021, 13, 1275. https://doi.org/10.3390/pharmaceutics13081275
Vanti G, Di Cesare Mannelli L, Micheli L, Cinci L, Grifoni L, Bergonzi MC, Ghelardini C, Bilia AR. The Anti-Arthritic Efficacy of Khellin Loaded in Ascorbyl Decanoate Nanovesicles after an Intra-Articular Administration. Pharmaceutics. 2021; 13(8):1275. https://doi.org/10.3390/pharmaceutics13081275
Chicago/Turabian StyleVanti, Giulia, Lorenzo Di Cesare Mannelli, Laura Micheli, Lorenzo Cinci, Lucia Grifoni, Maria Camilla Bergonzi, Carla Ghelardini, and Anna Rita Bilia. 2021. "The Anti-Arthritic Efficacy of Khellin Loaded in Ascorbyl Decanoate Nanovesicles after an Intra-Articular Administration" Pharmaceutics 13, no. 8: 1275. https://doi.org/10.3390/pharmaceutics13081275