Nanoradiopharmaceuticals Based on Alpha Emitters: Recent Developments for Medical Applications
Abstract
:1. Introduction
2. Alpha Emitters Used in TAT
2.1. Actinium-225 (225Ac)
2.2. Astatine-211 (211At)
2.3. Bismuth-213 (213Bi)
2.4. Lead-212 (212Pb)
2.5. Radium-223 (223Ra)
2.6. Thorium-226 (226Th)
2.7. Terbium-149 (149Tb)
3. Alpha Emitters in Medical Applications
4. Recoil Energy
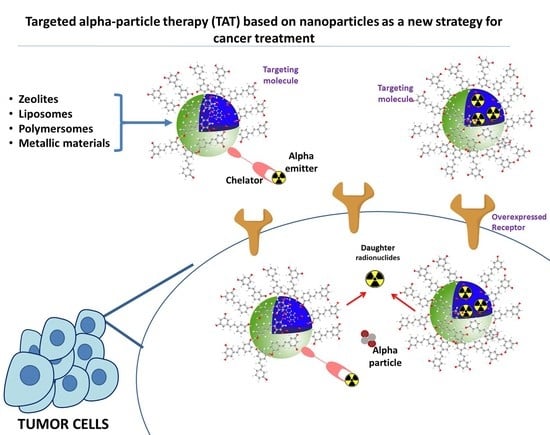
5. Strategies
5.1. Encapsulation of Radionuclides in a Nanocarrier
5.2. Targeting Vehicle
5.3. Local Administration
6. Chelating Agents
6.1. 225Ac
6.2. 212Pb
6.3. 212/213Bi
6.4. 223Ra
6.5. 149Tb
6.6. 226/227Th
6.7. 211At
7. Targeting Vectors
8. Nanoradiopharmaceuticals Based on Alpha Emitters
| Radionuclide | Nanoparticle (Type) | Retention/Release of Daughter Nuclides | Labeling Yield | Size | Ref |
|---|---|---|---|---|---|
| 225Ac | 225Ac(La(225Ac)PO4) |
| 66% | 3–5 nm | [74] |
| Multilayered {La0.5Gd0.5}PO4@GdPO4@Au doped with 225Ac |
| ~76% | 27 nm | [31] | |
| Multilayer {La0.5Gd0.5}(225Ac)PO4@4GdPO4 shell@Au |
| --- | --- | [77] | |
| Multilayer {Gd0.75La0.25}(225Ac)PO4@4 LaPO4 shell@Au |
| 76% | 19.9 ± 6.5 nm | [78] | |
| Gadolinium vanadate (GdVO4) |
| --- | --- | [24] | |
| Gadolinium vanadate core (Gd(225Ac)VO4) and core + 2 shell (Gd(225Ac)VO4/2GdVO4) |
| Yield of 225Ac was 77.1 ± 13.2% and 96.6 ± 1.6% for core and core + 2 shell, respectively Yield of 227Th was 62.9 ± 2.7% and 81.9 ± 1.2% for core and core + 2 shell, respectively | 3.6 ± 0.9 nm for core and 4.4 ± 1.0 nm for core + 2 shell | [79] | |
| Gd0.8Eu0.2VO4 core and core + 2 shells |
| Yield core was 41.1 ± 16.5%, shells increased the yield up to 55% | 6.1 ± 1.4 nm for core and 12.4 ± 2.0 nm for core + 2 shells | [80] | |
| Liposomes: Pegylated liposomes membrane charge (zwitterionic and cationic) |
| 6.4–10.0% | 200/400/650 nm | [11] | |
| Liposomes |
| 55–73% | 121 ± 6 nm | [81] | |
| Liposomes |
| 58.0–85.6% | 107 ± 2 nm | [82] | |
| Multivesicular liposomes (MUVELs) |
| --- | --- | [83] | |
| Polymersomes |
| 213Bi was 83 ± 75% (100 nm) 225Ac was 67 ± 0.8% (100 nm) | 100, 200, 400, and 800 nm | [28] | |
| Double-layered polymersomes |
| --- | 300–800 nm | [26] | |
| Polymersomes |
| 89 ± 0.6% | 100/200/400/800 nm | [84] | |
| Polymerosomes | --- | >90% for 111In and >64% for 225Ac | --- | [85] | |
| Polymerosomes |
| 54–59% | 97 ± 37 nm | [86] | |
| Fullerenes: 225Ac metallofullerene | --- | --- | --- | [87] | |
| TiO2 |
| 99.8 ± 2.1% | 25 nm | [88] | |
| Carbon nanotubes |
| --- | --- | [89] | |
| Carbon nanotubes | --- | ~95% | --- | [90] | |
| Lipid vehicle |
| 62.7 ± 14.6% | 106 ± 4 nm | [91] | |
| 223Ra | LnPO4 core and core + 2 shells NPs (Ln = La, Gd) |
| 91% for LaPO4 core + 2 shell | 3.4 nm for core and 6.3 nm for core + 2 shells | [75] |
| Hydroxyapatite (HA) |
| >95% | 15 nm | [92] | |
| Hydroxyapatite (nHAp) and titanium dioxide (nTiO2) |
| ~95% | --- | [93] | |
| Hydroxyapatite (HAP) | --- | 98% | Width up to 100 nm and length up to 500 nm | [94] | |
| Hydroxyapatite (HAP) | --- | 98% | 900–1000 μm | [95] | |
| Liposomes |
| 223Ra was 78 ± 6% 228Ac was 61 ± 8% | --- | [96] | |
| Liposomes: Pegylated liposomal doxorubicin (PLD) |
| 51–67% | 80 nm | [97] | |
| 223/224/225Ra-nanozeolite (NaA) |
| 99.9% | 30–70 nm | [22] | |
| 223Ra-labeled nanozeolite |
| 99.9% | 50–80 nm | [76] | |
| BaSO4 | --- | 20% | 140 nm | [98] | |
| Superparamagnetic iron oxide nanoparticles Fe3O4 SPIONS |
| 85–99% (PBS) | 4–26 nm | [99] | |
| Reduced graphite oxide |
| Sorption was 10% for 223Ra, 90% of 99mTc, 80–100% for 207Bi and 90Y | 4–6 nm | [100] | |
| Polyoxopalladates (Pd-POM): [224Ra]Na-a(Ra)Pd15 |
| --- | --- | [101] | |
| Calcium carbonate microparticles |
| >80% of 224Ra and 212Pb (daughter nuclide) | --- | [102] | |
| 211At | Silver core coated by PEO shell | --- | 50–97% | 18.3–34.7 nm | [73] |
| Ultrashort nanotubes |
| 77.7–91.3% | 20–50 nm in length and 1 nm diameter | [103] | |
| Gold nanoparticles (AuNPs) | --- | >99% | 5 and 15 nm | [104] | |
| Gold nanoparticles (AuNPs) | --- | >99% | 5 nm | [105] | |
| 212Pb | Liposomes | --- | 75% | --- | [106] |
| Liposomes |
| 90 ± 2% | --- | [107] | |
| Hydroxyapatite (HAP) | --- | --- | --- | [108] |
9. Discussion
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, Å.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted α particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Seidl, C. Radioimmunotherapy with α -particle-emitting radionuclides. Immunotherapy 2014, 6, 431–458. [Google Scholar] [CrossRef]
- Maguire, W.F.; McDevitt, M.R.; Smith-Jones, P.M.; Scheinberg, D.A. Efficient 1-step radiolabeling of monoclonal antibodies to high specific activity with 225Ac for a-particle radioimmunotherapy of cancer. J. Nucll Med. 2014, 55, 1492–1498. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tian, Z.; Rizvi, S.M.A.; Bander, N.H.; Allen, B.J. In vitro and preclinical targeted alpha therapy of human prostate cancer with Bi-213 labeled J591 antibody against the prostate specific membrane antigen. Prostate Cancer Prostatic Dis. 2002, 5, 36–46. [Google Scholar] [CrossRef]
- Ferrier, M.G.; Radchenko, V.; Wilbur, D.S. Radiochemical aspects of alpha emitting radionuclides for medical application. Radiochim Acta 2019, 107, 1065–1085. [Google Scholar] [CrossRef]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for targeted α therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of 225 Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Thorek, D.L.J.; Hashimoto, T.; Gondo, T.; Veach, D.R.; Sharma, S.K.; Kalidindi, T.M.; Abou, D.S.; Watson, P.A.; Beattie, B.J.; et al. Feed-forward alpha particle radiotherapy ablates androgen receptor-addicted prostate cancer. Nat. Commun. 2018, 9, 1629. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, U.; Jimenez, I.O.; Calzolai, L. A random walk approach to estimate the confinement of α -particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm. Chem. 2018, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted a-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Dosimetry estimate and empiric dose finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- Sofou, S.; Thomas, J.L.; Lin, H.Y.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Engineered liposomes for potential α-particle therapy of metastatic cancer. J. Nucl. Med. 2004, 45, 253–260. [Google Scholar]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD pamphlet No. 22 (Abridged): Radiobiology and dosimetry of α-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225 Actinium and 213 Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- Ballangrud, Å.M.; Yang, W.H.; Charlton, D.E.; McDevitt, M.R.; Hamacher, K.A.; Panageas, K.S.; Ma, D.; Bander, N.H.; Scheinberg, D.A.; Sgouros, G. Response of LNCaP spheroids after treatment with an α-particle emitter (213Bi)-labeled anti-prostate-specific membrane antigen antibody (J591). Cancer Res. 2001, 61, 2008–2014. [Google Scholar]
- K Kiess, A.P.; Minn, I.; Vaidyanathan, G.; Hobbs, R.F.; Josefsson, A.; Shen, C.; Brummet, M.; Chen, Y.; Choi, J.; Koumarianou, E.; et al. (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido)pentyl) ureido)-pentanedioic acid for PSMA-targeted α-particle radiopharmaceutical therapy. J. Nucl. Med. 2016, 57, 1569–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majkowska-pilip, A.; Gaw, W. Nanoparticles in Targeted Alpha Therapy. Nanomaterials 2020, 10, 1366. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted a-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Lacoeuille, F.; Arlicot, N.; Faivre-Chauvet, A. Un état des lieux des aspects radiopharmaceutiques et cliniques de la radiothérapie vectorisée par émetteurs alpha et bêta. Med. Nuclearie. 2018, 42, 32–44. [Google Scholar]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; Matthias, D.; Devoogdt, N. Targeted alpha therapy using short-lived alpha- particles and the promise of nanobodies as targeting vehicle. Expert Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayed, T.; Pilmé, J.; Tézé, D.; Bassal, F.; Barbet, J.; Chérel, M.; Champion, J.; Maurice, R.; Montavon, G.; Galland, N. 211At-labeled agents for alpha-immunotherapy: On the in vivo stability of astatine-agent bonds. Eur. J. Med. Chem. 2016, 116, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with224,225Ra for targeted alpha therapy. J. Nanopart. Res. 2013, 15, 2082. [Google Scholar] [CrossRef] [Green Version]
- De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A critical review of alpha radionuclide therapy-how to deal with recoiling daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Toro-González, M.; Dame, A.N.; Mirzadeh, S.; Rojas, J.V. Encapsulation and retention of 225Ac, 223Ra, 227Th, and decay daughters in zircon-type gadolinium vanadate nanoparticles. Radiochim. Acta 2020, 108, 967–977. [Google Scholar] [CrossRef]
- Silindir-gunay, M.; Karpuz, M.; Ozer, A.Y. Targeted Alpha Therapy and Nanocarrier Approach. Cancer Biother. Radiopharm. 2020, 35, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, L.; Schaart, D.R.; De Vries, D.; Morgenstern, A.; Bruchertseifer, F.; Denkova, A.G. Polymersomes as nano-carriers to retain harmful recoil nuclides in alpha radionuclide therapy: A feasibility study. Radiochim. Acta 2012, 100, 473–481. [Google Scholar] [CrossRef]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in targeted alpha-particle therapy. What we learned about recoils release from in vivo generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; de Kruijff, R.M.; Rol, A.; Thijssen, L.; Mendes, E.; Morgenstern, A.; Bruchertseifer, F.; Stuart, M.C.A.; Wolterbeek, H.T.; Denkova, A.G. Retention studies of recoiling daughter nuclides of 225Ac in polymer vesicles. Appl. Radiat. Isot. 2014, 85, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Shinto, A.S.; Kamaleshwaran, K.K.; Chakraborty, S.; Vyshakh, K.; Thirumalaisamy, S.G.; Karthik, S.; Nagaprabhu, V.N.; Vimalnath, K.V.; Das, T.; Banerjee, S. Radiosynovectomy of Painful Synovitis of Knee Joints Due to Rheumatoid Arthritis by Intra-Articular Administration of Lu-Labeled Hydroxyapatite Particulates: First Human Study and Initial Indian Experience. World J. Nucl. Med. 2015, 14, 81. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. Nuclear Instruments and Methods in Physics Research B SRIM—The stopping and range of ions in matter (2010). Nucl. Instrum. Methods Phys. Res. B 2010, 268, 1818–1823. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, M.F.; Woodward, J.; Boll, R.A.; Wall, J.S.; Rondinone, A.J.; Kennel, S.J.; Mirzadeh, S.; Robertson, J.D. Gold Coated Lanthanide Phosphate Nanoparticles for Targeted Alpha Generator Radiotherapy. PLoS ONE 2013, 8, e54531. [Google Scholar] [CrossRef] [Green Version]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2013, 43, 260–290. [Google Scholar] [CrossRef]
- Kerlin, M.G.F.; Radchenko, V. An Appendix of Radionuclides Used in Targeted Alpha Therapy. J Med. Imaging Radiat. Sci. 2019, 50, S58–S65. [Google Scholar]
- Behr, T.M.; Béhé, M.; Stabin, M.G.; Wehrmann, E.; Apostolidis, C.; Molinet, R.; Strutz, F.; Fayyazi, A.; Wieland, E.; Gratz, S.; et al. High-linear energy transfer (LET) α versus low-LET β emitters in radioimmunotherapy of solid tumors: Therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17-1a Fab’ fragments in a human colonic cancer model. Cancer Res. 1999, 59, 2635–2643. [Google Scholar]
- Gerwin, N.; Hops, C.; Lucke, A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 226–242. [Google Scholar] [CrossRef]
- Kim, Y.; Brechbiel, M.W. An overview of targeted alpha therapy. Tumor Biol. 2012, 33, 573–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, L.L.; Deal, K.A.; Dadachova, E.; Brechbiel, M.W. Synthesis, Conjugation, and Radiolabeling of a Novel Bifunctional Chelating Agent for 225 Ac Radioimmunotherapy Applications. Bioconjug. Chem. 2000, 11, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Thiele, A.N.A.; Brown, V.; Kelly, J.M.; Jermilova, U.; Macmillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Caterina, F.; Robertson, A.K.H.; Rodríguez, C.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. 2017, 56, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Ballangrud, Å.M.; Yang, W.; Palm, S.; Enmon, R.; Borchardt, P.E.; Pellegrini, V.A.; Mcdevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Alpha-Particle Emitting Atomic Generator (Actinium-225) -Labeled Trastuzumab ( Herceptin ) Targeting of Breast Cancer Spheroids: Efficacy versus HER2/neu Expression. Clin. Cancer Res. 2004, 10, 4489–4497. [Google Scholar] [CrossRef] [Green Version]
- Mcdevitt, M.R.; Ma, D.; Simon, J.; Frank, R.K.; Scheinberg, D.A. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002, 57, 841–847. [Google Scholar] [CrossRef]
- Song, H.; Hobbs, R.F.; Vajravelu, R.; Huso, D.L.; Esaias, C.; Apostolidis, C.; Morgenstern, A.; Sgouros, G. Radioimmunotherapy of Breast Cancer Metastases with α -Particle Emitter 225 Ac: Comparing Efficacy with 213 Bi and 90 Y. Cancer Res. 2009, 69, 8941–8949. [Google Scholar] [CrossRef] [Green Version]
- Bartos, B.; Kasperek, A.; Krajewski, S. Search of ligands suitable for Pb / 212 Bi in vivo generators. J. Radioanal. Nucl. Chem. 2013, 295, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruegg, C.L.; Anderson-Berg, W.T.; Brechbiel, M.W.; Mirzadeh, S.; Gansow, O.A.; Strand, M. Improved in Vivo Stability and Tumor Targeting of Bismuth-labeled Antibody. Cancer Res. 1990, 50, 4221–4226. [Google Scholar] [PubMed]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. 212Pb-radioimmunotherapy potentiates paclitaxel-induced cell killing efficacy by perturbing the mitotic spindle checkpoint. Br. J. Cancer 2013, 108, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Methodology for labeling proteins and peptides with lead-212 ( 212 Pb). Nucl. Med. Biol. 2013, 40, 592–599. [Google Scholar] [CrossRef] [Green Version]
- England, N.; Hospital, D. Tetraacetic acid (DOTA) -Bismuth-conjugated Anti-Tac Antibody for c -Emitter (212Bi) Therapy 1. Cancer Res. 1993, 53, 5683–5689. [Google Scholar]
- Song, H.A.; Kang, C.S.; Baidoo, K.E.; Milenic, D.E.; Chen, Y.; Dai, A.; Brechbiel, M.W.; Chong, H. Efficient Bifunctional Decadentate Ligand 3p- C -DEPA for Targeted r -Radioimmunotherapy Applications. Bioconjug. Chem. 2011, 22, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.; Song, H.A.; Ma, X.; Milenic, D.E.; Brady, E.D.; Lim, S.; Lee, H.; Baidoo, K.; Cheng, D.; Brechbiel, M.W. Novel Bimodal Bifunctional Ligands for Radioimmunotherapy and Targeted MRI. Bioconjug. Chem. 2008, 19, 1439–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miederer, M.; Mcdevitt, M.R.; Sgouros, G.; Kramer, K.; Cheung, N.V.; Scheinberg, D.A. Pharmacokinetics, Dosimetry, and Toxicity of the Targetable Atomic Generator, 225Ac-HuM195, in Nonhuman Primates. J. Nucl. Med. 2004, 45, 129–137. [Google Scholar]
- Beyer, G.J.; Miederer, M.; Vranješ-Durić, S.; Čomor, J.J.; Künzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F. Targeted alpha therapy in vivo: Direct evidence for single cancer cell kill using 149Tb-rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554. [Google Scholar] [CrossRef]
- Chong, H.; Song, H.A.; Kang, C.S.; Le, T.; Sun, X.; Dadwal, M.; Lee, H.; Lan, X.; Chen, Y.; Dai, A. ChemComm COMMUNICATION A highly effective bifunctional ligand for radioimmunotherapy applications. Chem. Commun. 2011, 47, 5584–5586. [Google Scholar] [CrossRef]
- Wilbur, D.S. The Radiopharmaceutical Chemistry of Alpha-Emitting Radionuclides. In Radiopharmaceutical Chemistry; Lewis, J., Windhorst, A., Zeglis, B., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Gott, M.; Steinbach, J.; Mamat, C. The Radiochemical and Radiopharmaceutical Applications of Radium. Open Chem. 2016, 14, 118–129. [Google Scholar] [CrossRef]
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; Van Der Walt, N.T.; Türler, A.; Schibli, R. A unique matched quadruplet of terbium radioisotopes for PET and SPECT and for α- and β-radionuclide therapy: An in vivo proof-of-concept study with a new receptor-targeted folate derivative. J. Nucl. Med. 2012, 53, 1951–1959. [Google Scholar] [CrossRef] [Green Version]
- Larsen, R.H.; Borrebaek, J.; Dahle, J.; Melhus, K.B.; Krogh, C.; Valan, M.H.; Bruland, Ø.S. Preparation of TH227-labeled radioimmunoconjugates, assessment of serum stability and antigen binding ability. Cancer Biother. Radiopharm. 2007, 22, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Ramdahl, T.; Bonge-hansen, H.T.; Ryan, O.B.; Larsen, Å.; Herstad, G.; Sandberg, M.; Bjerke, R.M.; Grant, D.; Ellen, M.; Cuthbertson, A.S. Efficient Chelator for Complexation of Thorium-227. Bioorg. Med. Chem. Lett. 2016, 26, 4318–4321. [Google Scholar] [CrossRef] [Green Version]
- Wilbur, D.S.; Chyan, M.; Nakamae, H.; Chen, Y.; Hamlin, D.K.; Santos, E.B.; Kornblit, B.T.; Sandmaier, B.M. Reagents for Astatination of Biomolecules. 6. An Intact Antibody Conjugated with a Maleimido-closo-Decaborate(2-) Reagent via Sulfhydryl Groups Had Considerably Higher Kidney Concentrations than the Same Antibody Conjugated with an Isothiocyanato-closo- D. Bioconjug. Chem. 2012, 23, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Zalutsky, M.; Vaidyanathan, G. Astatine-211-Labeled Radiotherapeutics An Emerging Approach to Targeted Alpha-Particle Radiotherapy. Curr. Pharm. Des. 2005, 6, 1433–1455. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with α-particle-emitting 211 At: Treatment of recurrent brain tumor patients with 211 At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederkrantz, E.; Andersson, H.; Bernhardt, P.; Bäck, T.; Hultborn, R.; Jacobsson, L.; Jensen, H.; Lindegren, S.; Ljungberg, M.; Magnander, T.; et al. Absorbed Doses and Risk Estimates of 211At-MX35 F(ab’)2 in Intraperitoneal Therapy of Ovarian Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 569–576. [Google Scholar] [CrossRef]
- Li, Y.; Hamlin, D.K.; Chyan, M.K.; Wong, R.; Dorman, E.F.; Emery, R.C.; Woodle, D.R.; Manger, R.L.; Nartea, M.; Kenoyer, A.L.; et al. CGMP production of astatine-211-labeled anti-CD45 antibodies for use in allogeneic hematopoietic cell transplantation for treatment of advanced hematopoietic malignancies. PLoS ONE 2018, 13, e0205135. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Cell killing mechanisms and impact on gene expression by gemcitabine and 212Pb-trastuzumab treatment in a disseminated i.p. tumor model. PLoS ONE 2016, 11, e0159904. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Baidoo, K.E.; Kim, Y.S.; Barkley, R.; Brechbiel, M.W. Targeted α-Particle Radiation Therapy of HER1-Positive Disseminated Intraperitoneal Disease: An Investigation of the Human Anti-EGFR Monoclonal Antibody, Panitumumab. Transl. Oncol. 2017, 10, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Kim, Y.S.; Baidoo, K.E.; Wong, K.J.; Barkley, R.; Delgado, J.; Brechbiel, M.W. Exploration of a F(ab′)2 Fragment as the Targeting Agent of α-Radiation Therapy: A Comparison of the Therapeutic Benefit of Intraperitoneal and Intravenous Administered Radioimmunotherapy. Cancer Biother. Radiopharm. 2018, 33, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuci, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213 Bi-substance P analogue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1636–1644. [Google Scholar] [CrossRef] [Green Version]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1100.68. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, C.; Graham, P.; Rizvi, S.; Song, E.; Goldsmith, H.; Bosserhoff, A.; Morgenstern, A.; Apostolidis, C.; Reisfeld, R.; Allen, B.J.; et al. Interim Analysis of Toxicity and Response in Phase 1 Trial of Systemic RIB. Cancer Biol. Ther. 2007, 6, 846852. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W. Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Schmidt, K.; Afshar-oromieh, A.; Bruchertseifer, F.; Rathke, H.; Morgenstern, A.; Haberkorn, U.; Giesel, F.L. Targeted alpha therapy of mCRPC: Dosimetry estimate of 213 Bismuth-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Hagemann, U.B.; Wickstroem, K.; Wang, E.; Shea, A.O.; Sponheim, K.; Karlsson, J.; Bjerke, R.M.; Ryan, O.B.; Cuthbertson, A.S. In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2016, 15, 2422–2432. [Google Scholar] [CrossRef] [Green Version]
- Hagemann, U.B.; Mihaylova, D.; Uran, S.R.; Borrebaek, J. Targeted alpha therapy using a novel CD70 targeted thorium-227 conjugate in in vitro and in vivo models of renal cell carcinoma. Oncotarget 2017, 8, 56311–56326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuc, J. Astatination of nanoparticles containing silver as possible carriers of 211At. Appl. Radiat. Isot. 2006, 64, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO 4 Nanoparticles Doped with Actinium-225 that Partially Sequester Daughter Radionuclides. Bioconjug. Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef]
- Rojas, J.V.; Woodward, J.D.; Chen, N.; Rondinone, A.J.; Castano, C.H.; Mirzadeh, S. Synthesis and characterization of lanthanum phosphate nanoparticles as carriers for 223Ra and 225Ra for targeted alpha therapy. Nucl. Med. Biol. 2015, 42, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska, A.; Sylwia, M.; Majkowska-pilip, A.; Wójciuk, G.; Edyta, C.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with Ra for targeted alpha therapy. Nucl Med Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef]
- McLaughlin, M.F.; Robertson, D.; Pevsner, P.H.; Wall, J.S.; Mirzadeh, S.; Kennel, S.J. LnPO4 nanoparticles doped with Ac-225 and sequestered daughters for targeted alpha therapy. Cancer Biother. Radiopharm. 2014, 29, 34–41. [Google Scholar] [CrossRef]
- Woodward, J.; Ridge, O.; Boll, R.; Ridge, O.; Rondinone, A.; Ridge, O.; Mirzadeh, S.; Ridge, O. Gold-coated lanthanide phosphate nanoparticles for an 225 Ac in vivo. Radiochim. Acta 2013, 101, 595–600. [Google Scholar]
- Toro-González, M.; Dame, A.N.; Mirzadeh, S.; Rojas, J.V. Gadolinium vanadate nanocrystals as carriers of α-emitters (225Ac, 227Th) and contrast agents. J. Appl. Phys. 2019, 125, 214901. [Google Scholar] [CrossRef]
- Toro-González, M.; Copping, R.; Mirzadeh, S.; Rojas, J.V. Multifunctional GdVO4:Eu core-shell nanoparticles containing 225Ac for targeted alpha therapy and molecular imaging. J. Mater. Chem. B 2018, 6, 7985–7997. [Google Scholar] [CrossRef]
- Chang, M.; Seideman, J.; Sofou, S. Enhanced Loading Efficiency and Retention of 225Ac in Rigid Liposomes for Potential Targeted Therapy of Micrometastases. Bioconjug. Chem. 2008, 19, 1274–1282. [Google Scholar] [CrossRef]
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular α-particle therapy of cancer. J. Nuc.l Med. 2014, 55, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Sofou, S.; Kappel, B.J.; Jaggi, J.S.; Mcdevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Enhanced Retention of the r -Particle-Emitting Daughters of Actinium-225 by Liposome Carriers. Bioconjug. Chem. 2007, 18, 2061–2067. [Google Scholar] [CrossRef] [Green Version]
- De Kruij, R.M.; Drost, K.; Thijssen, L.; Morgenstern, A.; Bruchertseifer, F.; Lathouwers, D. Ac daughter retention in InPO 4 containing polymersomes. Appl. Radiat. Isot. 2017, 128, 183–189. [Google Scholar] [CrossRef]
- de Kruijff, R.M.; van der Meer, A.J.G.M.; Windmeijer, C.A.A.; Kouwenberg, J.J.M.; Morgenstern, A.; Bruchertseifer, F.; Sminia, P.; Denkova, A.G. The therapeutic potential of polymersomes loaded with 225Ac evaluated in 2D and 3D in vitro glioma models. Eur. J. Pharm. Biopharm. 2018, 127, 85–91. [Google Scholar] [CrossRef]
- De Kruijff, R.M.; Raavé, R.; Kip, A.; Morgenstern, A. The in vivo fate of 225 Ac daughter nuclides using polymersomes as a model carrier. Sci. Rep. 2019, 9, 11671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, K.; Haba, H.; Sueki, K.; Tsukada, K.; Asai, M.; Toyoshima, A.; Nagame, Y.; Katada, M. 225Ac metallofullerene: Toward 225Ac nanogenerator in fullerene. Chem. Lett. 2009, 38, 978–979. [Google Scholar] [CrossRef]
- Edyta, C.; Pruszynski, M.; Majkowska-pilip, A.; Sylwia, M. Functionalized TiO 2 nanoparticles labelled with 225 Ac for targeted alpha radionuclide therapy. J. Nanopart.Res. 2018, 20, 83. [Google Scholar]
- Mulvey, J.J.; Villa, C.H.; McDevitt, M.R.; Escorcia, F.E.; Casey, E.; Scheinberg, D.A. Self-assembly of carbon nanotubes and antibodies on tumours for targeted amplified delivery. Nat. Nanotechnol. 2013, 8, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, M.L.; Villa, C.H.; Ananta, J.S.; Law, J.J.; Scheinberg, D.A.; Wilson, L.J. Encapsulation of a-particle-emitting 225Ac3+ ions within carbon nanotubes. J. Nucl. Med. 2015, 56, 897–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sempkowski, M.; Zhu, C.; Menzenski, M.Z.; Kevrekidis, I.G.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Sticky Patches on Lipid Nanoparticles Enable the Selective Targeting and Killing of Untargetable Cancer Cells. Langmuir 2016, 32, 8329–8338. [Google Scholar] [CrossRef]
- Kozempel, J.; Vlk, M. Prospective carriers of Ra for targeted alpha particle therapy. J. Radioanal. Nucl. Chem. 2014, 304, 443–447. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Vlk, M.; Kozempel, J. Study of 223ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of ph. RSC Adv. 2020, 10, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Vasiliev, A.N. Hydroxyapatite particles as carriers for 223Ra. J. Radioanal. Nucl. Chem. 2016, 311, 1–7. [Google Scholar] [CrossRef]
- Severin, A.V.; Vasiliev, A.N.; Gopin, A.V.; Vlasova, I.E.; Chernykh, E.V. Dynamics of Sorption—Desorption of 223Ra Therapeutic α-Emitter on Granulated Hydroxyapatite. Radiochemistry 2019, 61, 339–346. [Google Scholar] [CrossRef]
- Henriksen, G.; Schoultz, B.W.; Michaelsen, T.E.; Bruland, Ø.S.; Larsen, R.H. Sterically stabilized liposomes as a carrier for α-emitting radium and actinium radionuclides. Nucl. Med. Biol. 2004, 31, 441–449. [Google Scholar] [CrossRef]
- Jonasdottir, T.J.; Fisher, D.R.; Borrebæk, J.; Bruland, Ø.S.; Larsen, R.H. First in vivo evaluation of liposome-encapsulated 223Ra as a potential alpha-particle-emitting cancer therapeutic agent. Anticancer Res. 2006, 26, 2841–2848. [Google Scholar]
- Reissig, F.; Hübner, R.; Steinbach, J.; Pietzsch, H.J.; Mamat, C. Facile preparation of radium-doped, functionalized nanoparticles as carriers for targeted alpha therapy. Inorg. Chem. Front. 2019, 6, 1341–1349. [Google Scholar] [CrossRef]
- Kukleva, E.; Mic, P.; Mokhodoeva, O.; Vlk, M.; Ma, E.; Miroslav, S. Study of 223 Ra uptake mechanism by Fe 3 O 4 nanoparticles: Towards new prospective theranostic SPIONs. J. Nanopart. Res. 2016, 18, 301. [Google Scholar]
- Kazakov, A.G.; Garashchenko, B.L.; Yakovlev, R.Y.; Vinokurov, S.E.; Kalmykov, S.N.; Myasoedov, B.F. An experimental study of sorption/desorption of selected radionuclides on carbon nanomaterials: A quest for possible applications in future nuclear medicine. Diam. Relat. Mater. 2020, 104, 107752. [Google Scholar] [CrossRef]
- Gott, M.; Yang, P.; Kortz, U.; Stephan, H.; Pietzsch, H.J.; Mamat, C. A 224Ra-labeled polyoxopalladate as a putative radiopharmaceutical. Chem. Commun. 2019, 55, 7631–7634. [Google Scholar] [CrossRef] [PubMed]
- Westrøm, S.; Malenge, M.; Jorstad, I.S.; Napoli, E.; Bruland, Ø.S.; Bønsdorff, T.B.; Larsen, R.H. Ra-224 labeling of calcium carbonate microparticles for internal α-therapy: Preparation, stability, and biodistribution in mice. J. Label Compd. Radiopharm. 2018, 61, 472–486. [Google Scholar] [CrossRef] [Green Version]
- Hartman, K.B.; Hamlin, D.K.; Wilbur, D.S.; Wilson, L.J. 211AtCl@US-tube nanocapsules: A new concept in radiotherapeutic-agent design. Small 2007, 3, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Dziawer, L.; Koźmiński, P.; Mȩczyńska-Wielgosz, S.; Pruszyński, M.; Łyczko, M.; Wąs, B.; Celichowski, G.; Grobelny, J.; Jastrzȩbski, J.; Bilewicz, A. Gold nanoparticle bioconjugates labelled with 211At for targeted alpha therapy. RSC Adv. 2017, 7, 41024–41032. [Google Scholar] [CrossRef] [Green Version]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-modified gold nanoparticles labeled with 211 At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Le Du, A.; Mougin-Degraef, M.; Botosoa, E.; Rauscher, A.; Chauvet, A.F.; Barbet, J.; Montavon, G. In vivo 212Pb/212Bi generator using indium-DTPA-tagged liposomes. Radiochim. Acta 2011, 99, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, G.; Schoultzt, B.W.; Hoff, P.; Larsen, R.H. Potential in vivo generator for alpha-particle therapy with 212Bi: Presentation of a system to minimize escape of daughter nuclide after decay of 212Pb to 212Bi. Radiochim. Acta 2003, 91, 109–113. [Google Scholar] [CrossRef]
- Severin, A.V.; Orlova, M.A.; Shalamova, E.S.; Egorov, A.V.; Sirotin, M.A. Nanohydroxyapatite and its textures as potential carriers of promising short-lived lead isotopes. Russ. Chem. Bull. 2019, 68, 2197–2204. [Google Scholar] [CrossRef]







| Nuclide | t1/2 | Main Emissions | Energy (MeV) | Energy Recoil (KeV) | Range (µm) | Daughters | t1/2 | Decay | Energy (MeV) |
|---|---|---|---|---|---|---|---|---|---|
| 227Th | 18.7 d | α | 6 | --- | 50–70 | 223Ra | |||
| 225Ac | 10 d | α | 5.8 | --- | 50–90 | 221Fr | 4.8 m | A | 7 |
| γ | 0.218 | ||||||||
| 105 | 217At | 32.3 ms | A | 7 | |||||
| 116 | 213Bi | 45.6 m | A | 6 | |||||
| β− | 0.444 | ||||||||
| γ | 0.440 | ||||||||
| 132 | 213Po | 4.2 μs | A | 8 | |||||
| 209Tl | 2.2 m | β− | 0.659 | ||||||
| 160 | 209Pb | 3.5 h | β− | 0.198 | |||||
| 209Bi | Stable | --- | --- | ||||||
| 223Ra | 11.4 d | α | 5.7 | 108.4 | 50–70 | 219Ra | 3.96 s | A | 6.8 |
| 126 | 215Po | 1.78 ms | A | 7.4 | |||||
| 140 | 211Pb | 36.1 m | β− | --- | |||||
| 211Bi | 2.13 m | A | 6.6 | ||||||
| β− | --- | ||||||||
| 211Po | 0.516 s | A | --- | ||||||
| 128 | 207Tl | 4.77 m | β− | --- | |||||
| 207Pb | Stable | --- | --- | ||||||
| 213Bi | 45.6 m | α | 6 | 50–90 | 132 | 213Po | 4.2 μs | A | 8 |
| β− | 0.444 | 209Tl | 2.2 m | β− | 0.659 | ||||
| γ | 0.440 | 209Pb | 3.5 h | β− | 0.198 | ||||
| 209Bi | Stable | --- | --- | ||||||
| 212Bi | 60.6 m | α | 6.1 | 40–100 | --- | 212Po | 0.3 µs | A | --- |
| β− | 208Tl | 3.1 m | β− | --- | |||||
| 208Pb | Stable | --- | --- | ||||||
| 211At | 7.2 h | α | 5.9 | 55–80 | 116 | 207Bi | 33.4 y | β− | |
| EC | 211Po | 0.516 s | A | --- | |||||
| 207Pb | Stable | --- | --- |
| DOTA and Bifunctional Derivatives | Mainly Alpha Radionuclides | Ref | |
|---|---|---|---|
| DOTA: 1,4,7,10-tetra-azacyclododecane-1,4,7,10-tetraacetic acid |  | [32,36,37,38,39,40,41,42,43] | |
| DOTAGA-anhydride |  | 212Pb2+, 225Ac3+, 212/213Bi3+, 223Ra2+, 149Tb, 226/227Th | |
| DOTA-NHS-ester |  | ||
| DOTAGA, R = amide, DOTA-NHS-ester |  | ||
| p-SCN-Bn-DOTA (C-DOTA) |  | ||
| DOTA derivatives TCMC, 3p-C-DEPA, and bifunctional derivatives | |||
| 3p-C-DEPA: 2-[(carboxymethyl)]-[5-(4-nitrophenyl-1-[4,7,10-tris-(carboxymethyl)- 1,4,7,10-tetraazacyclododecan-1-yl]pentan-2-yl)-amino]acetic acid |  | [32,44,45,46,47] | |
| 3p-C-DEPA-NCS |  | ||
| TCMC, 1,4,7,10-tetrakis(carbamoylmethyl)- l,4,7,10-tetraazacyclododecane |  | 212Pb2+,212/213Bi3+ | |
| p-SCN-Bn-TCMC |  | ||
| NOTA, NETA, TACN-TM, and bifunctional derivatives | |||
| NOTA: 1,4,7-triazacyclononane-1,4,7-triacetic acid |  | [32,43,48] | |
| p-SCN-Bn-NOTA (C-NOTA) |  | ||
| NETA: {4-[2-(bis-carboxymethylamino)-ethyl]-7-carboxymethyl-[1,4,7]triazonan-1-yl}-acetic acid |  | ||
| C-NE3TA-NCS |  | 212/213Bi3+, 212Pb2+ | |
| 3p-C-NETA |  | ||
| C-NETA-NCS |  | ||
| DTPA, bifunctional derivatives, and others | |||
| DTPA: diethylenetriaminepentaacetic acid |  | [5,32,36,41,42,49,50] | |
| p-SCN-Bn-CHX-A″-DTPA |  | ||
| p-SCN-Bn-1B-DTPA |  | ||
| CHX-A”-DTPA, 2-(p-isothiocyanatobenzyl)- cyclohexyldiethylenetriaminepentaacetic acid |  | 212/213Bi3+, 225Ac3+, 223Ra2+, 149Tb, 226/227Th | |
| p-SCN-Bn-1B4M-DTPA |  | ||
| (Me-2,3-HOPO)4-Bn-NCS |  | 226/227Th | |
| HEHA, PEPA, and bifunctional derivatives | |||
| HEHA: 1,4,7,10,13,16-hexaazacyclo-hexadecane-N′,N″,N‴,N,N⁗,N″″′-hexaacetic acid |  | [32,36,37,38] | |
| p-SCN-Bn-HEHA (C-HEHA) |  | ||
| PEPA: 1,4,7,10,13-pentaazacyclopentadecane-N,N′,N″,N‴,N⁗-pentaacetic acid |  | ||
| p-SCN-Bn-PEPA (C-PEPA) |  | ||
| MACROPA |  | 225Ac3+ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo-Nolasco, M.; Morales-Avila, E.; Cruz-Nova, P.; Katti, K.V.; Ocampo-García, B. Nanoradiopharmaceuticals Based on Alpha Emitters: Recent Developments for Medical Applications. Pharmaceutics 2021, 13, 1123. https://doi.org/10.3390/pharmaceutics13081123
Trujillo-Nolasco M, Morales-Avila E, Cruz-Nova P, Katti KV, Ocampo-García B. Nanoradiopharmaceuticals Based on Alpha Emitters: Recent Developments for Medical Applications. Pharmaceutics. 2021; 13(8):1123. https://doi.org/10.3390/pharmaceutics13081123
Chicago/Turabian StyleTrujillo-Nolasco, Maydelid, Enrique Morales-Avila, Pedro Cruz-Nova, Kattesh V. Katti, and Blanca Ocampo-García. 2021. "Nanoradiopharmaceuticals Based on Alpha Emitters: Recent Developments for Medical Applications" Pharmaceutics 13, no. 8: 1123. https://doi.org/10.3390/pharmaceutics13081123