In Vitro Wound-Healing Properties of Water-Soluble Terpenoids Loaded on Halloysite Clay
Abstract
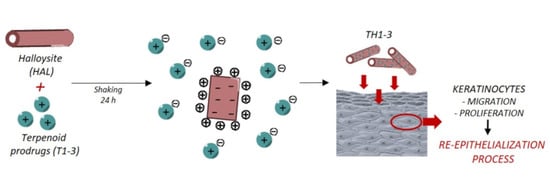
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Equilibrium Studies
2.3. Solid-State Characterization
2.3.1. X-ray Powder Diffraction (XRPD)
2.3.2. Thermal Analysis
2.3.3. Fourier Transform Infrared (FTIR)
2.4. In Vitro Release Studies
2.5. In Vitro Wound-Healing Assays
2.6. Time-Kill Assays
3. Results and Discussion
3.1. TH1-3 Characterization
3.2. Wound Healing and Antimicrobial Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- García-Villén, F.; Carazo, E.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Cerezo, P.; Viseras, C.; Aguzzi, C. Clay minerals in drug delivery systems. In Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–166. [Google Scholar]
- Khatoon, N.; Chu, M.Q.; Zhou, C.H. Nanoclay-based drug delivery systems and their therapeutic potentials. J. Mater. Chem. B 2020, 8, 7335–7351. [Google Scholar] [CrossRef] [PubMed]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef]
- Aguzzi, C.; Sandri, G.; Cerezo, P.; Viseras, C. Health and medical applications of tubular clay minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 7, pp. 708–725. [Google Scholar]
- Viseras, M.T.; Aguzzi, C.; Cerezo, P.; Cultrone, G.; Viseras, C. Supramolecular structure of 5-aminosalycilic acid/halloysite composites. J. Microencapsul. 2009, 26, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Lvov, Y. Halloysite for Controllable Loading and Release. In Developments in Clay Science, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 554–605. [Google Scholar]
- Can Baser, K. Biological and Pharmacological Activities of Carvacrol and Carvacrol Bearing Essential Oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The Use of Some Clay Minerals as Natural Resources for Drug Carrier Applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fizir, M.; Dramou, P.; Dahiru, N.S.; Ruya, W.; Huang, T.; He, H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta 2018, 185, 389. [Google Scholar] [CrossRef]
- Hendessi, S.; Sevinis, E.B.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Antibacterial sustained-release coatings from halloysite nanotubes/waterborne polyurethanes. Prog. Org. Coat. 2016, 101, 253–261. [Google Scholar] [CrossRef]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial carvacrol-containing polypropylene films: Composition, structure and function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Shemesh, R.; Goldman, D.; Krepker, M.; Danin-Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. LDPE/clay/carvacrol nanocomposites with prolonged antimicrobial activity. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Marinelli, L.; Fornasari, E.; Eusepi, P.; Ciulla, M.; Genovese, S.; Epifano, F.; Fiorito, S.; Turkez, H.; Örtücü, S.; Mingoia, M.; et al. Carvacrol prodrugs as novel antimicrobial agents. Eur. J. Med. Chem. 2019, 178, 515–529. [Google Scholar] [CrossRef]
- Eusepi, P.; Marinelli, L.; García-Villén, F.; Borrego-Sánchez, A.; Cacciatore, I.; Di Stefano, A.; Viseras, C. Carvacrol prodrugs with antimicrobial activity loaded on clay nanocomposites. Materials 2020, 13, 1793. [Google Scholar] [CrossRef] [Green Version]
- Eusepi, P.; Marinelli, L.; Borrego-Sánchez, A.; García-Villén, F.; Khalifa Rayhane, B.; Cacciatore, I.; Viseras, C.; Di Stefano, A. Nano-delivery systems based on carvacrol prodrugs and fibrous clays. J. Drug Deliv. Sci. Technol. 2020, 58, 101815. [Google Scholar] [CrossRef]
- Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Aguzzi, C.; Viseras, C.; Sainz-Díaz, C.I.; Cerezo, P. Assessment of halloysite nanotubes as vehicles of isoniazid. Colloids Surf. B Biointerfaces 2017, 160, 337–344. [Google Scholar] [CrossRef]
- Massaro, M.; Noto, R.; Riela, S. Past, Present and Future Perspectives on Halloysite Clay Minerals. Molecules 2020, 25, 4863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.T.; Chang, P.H.; Tsai, Y.; Li, Z. Halloysite nanotubes as a carrier for the uptake of selected pharmaceuticals. Microporous Mesoporous Mater. 2016, 220, 298–307. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why does vacuum drive to the loading of halloysite nanotubes? The key role of water confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Perini, I.; Ridi, F.; Baglioni, P. Improving the properties of antifouling hybrid composites: The use of Halloysites as nano-containers in epoxy coatings. Colloid Surf. A Physicochem. Eng. Asp. 2021, 623, 126779. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, A. Alkali activation of halloysite for adsorption and release of ofloxacin. Appl. Surf. Sci. 2013, 287, 54–61. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Ripoll, L.; Hidalgo, M.; Lopez-Martinez, J.; Balart, R. Characterization of selectively etched halloysite nanotubes by acid treatment. Appl. Surf. Sci. 2017, 422, 616–625. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Carazo, E.; Aguzzi, C.; Viseras, C.; Sainz-Díaz, C.I. Biopharmaceutical improvement of praziquantel by interaction with montmorillonite and sepiolite. Appl Clay Sci. 2018, 160, 173–179. [Google Scholar] [CrossRef]
- Duce, C.; Della Porta, V.; Bramanti, E.; Campanella, B.; Spepi, A.; Tiné, M.R. Loading of halloysite nanotubes with BSA, α-Lac and β-Lg: A Fourier transform infrared spectroscopic and thermogravimetric study. Nanotechnology 2017, 28, 055706. [Google Scholar] [CrossRef] [Green Version]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole. ACS Appl. Mater. Interfaces 2009, 1, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.R.; Deasy, P.B. Use of coated microtubular halloysite for the sustained release of diltiazem hydrochloride and propranolol hydrochloride. Int. J. Pharm. 2003, 253, 145–157. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.; Nassir, M.; HAl-Amiery, A.A. Impact of sulfuric acid treatment of halloysite on physico-chemic property modification. Materials 2016, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies. J. Mol. Struct. 2015, 1084, 16–22. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, S.; Clinton, A.; Fowler, H.; Everett, J.; Horswill, A.R.; Rumbaugh, K.P. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect Immun. 2014, 82, 4718–4728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonetti, O.; Cirioni, O.; Mocchegiani, F.; Cacciatore, I.; Silvestri, C.; Baldassarre, L.; Orlando, F.; Castelli, P.; Provinciali, M.; Vivarelli, M.; et al. The efficacy of the quorum sensing inhibitor FS8 and tigecycline in preventing prosthesis biofilm in an animal model of staphylococcal infection. Int. J. Mol. Sci. 2013, 14, 16321–16332. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Dielubanza, E.; Maisel, A.; Leung, K.; Mustoe, T.; Hong, S.; Galiano, R. Staphylococcus aureus impairs cutaneous wound healing by activating the expression of a gap junction protein, connexin-43 in keratinocytes. Cell. Mol. Life Sci. 2021, 78, 935–947. [Google Scholar] [CrossRef]
- Buch, P.J.; Chai, Y.; Goluch, E.D. Bacterial chatter in chronic wound infections. Wound Repair Regen. 2021, 29, 106–116. [Google Scholar] [CrossRef] [PubMed]











| CAR | T1 | T2 | T3 | |
|---|---|---|---|---|
| Molecular Weight (g/mol) | 150.22 | 243.73 | 257.76 | 257.76 |
| Water Solubility (mg/mL) a | 0.11 | 586.87 | 190.63 | 479.33 |
| LogP b | 2.82 | 2.17 | 2.53 | 2.43 |
| Percentage Wound Width | ||
|---|---|---|
| T = 24 b | T = 48 b | |
| Spontaneous | 88 (±5) | 16 (±6) |
| HAL | 35 (±13) | 0 (±7) |
| T1 | 58 (±17) | 0 (±5) |
| T2 | 62 (±8) | 14 (±3) |
| T3 | 62 (±12) | 27 (±8) |
| TH1 | 73 (±17) | 0 (±5) |
| TH2 | 70 (±8) * | 0 (±3) |
| TH3 | 55 (±14) * | 0 (±1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinelli, L.; Cacciatore, I.; Eusepi, P.; Dimmito, M.P.; Di Rienzo, A.; Reale, M.; Costantini, E.; Borrego-Sánchez, A.; García-Villén, F.; Viseras, C.; et al. In Vitro Wound-Healing Properties of Water-Soluble Terpenoids Loaded on Halloysite Clay. Pharmaceutics 2021, 13, 1117. https://doi.org/10.3390/pharmaceutics13081117
Marinelli L, Cacciatore I, Eusepi P, Dimmito MP, Di Rienzo A, Reale M, Costantini E, Borrego-Sánchez A, García-Villén F, Viseras C, et al. In Vitro Wound-Healing Properties of Water-Soluble Terpenoids Loaded on Halloysite Clay. Pharmaceutics. 2021; 13(8):1117. https://doi.org/10.3390/pharmaceutics13081117
Chicago/Turabian StyleMarinelli, Lisa, Ivana Cacciatore, Piera Eusepi, Marilisa Pia Dimmito, Annalisa Di Rienzo, Marcella Reale, Erica Costantini, Ana Borrego-Sánchez, Fátima García-Villén, César Viseras, and et al. 2021. "In Vitro Wound-Healing Properties of Water-Soluble Terpenoids Loaded on Halloysite Clay" Pharmaceutics 13, no. 8: 1117. https://doi.org/10.3390/pharmaceutics13081117