Effect of Storage Humidity on Physical Stability of Spray-Dried Naproxen Amorphous Solid Dispersions with Polyvinylpyrrolidone: Two Fluid Nozzle vs. Three Fluid Nozzle
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
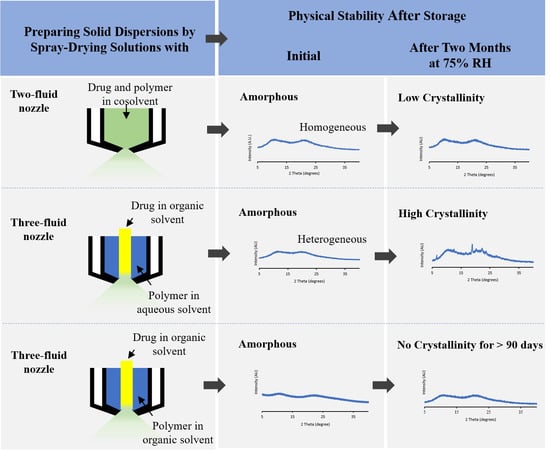
2.2. Formation of Solid Dispersion via Spray Drying
2.3. Storage Stability Study
2.4. Powder X-ray Diffraction
2.5. Thermogravimetry
2.6. Dynamic Vapor Sorption (DVS)
2.7. Scanning Electron Microscopy (SEM)
2.8. Polarized Light Microscopy
2.9. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
2.10. X-ray Photoelectron Spectroscopy (XPS)
2.11. Solid-State NMR Spectroscopy
2.12. Statistical Analysis
3. Results
3.1. Physical Characterization of ASDs upon Spray-Drying
3.2. Physical Stability of ASDs upon Storage
3.3. Drug-Polymer Surface Distribution
3.4. Homogeneity of Drug and Polymer Determined as by Solid-State NMR (ssNMR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jermain, S.V.; Brough, C.; Williams, R.O., III. Amorphous Solid Dispersions and Nanocrystal Technologies for Poorly Water-Soluble Drug Delivery–An Update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Fahr, A.; Liu, X. Drug Delivery Strategies for Poorly Water-Soluble Drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Van Hoogevest, P.; Liu, X.; Fahr, A. Drug Delivery Strategies for Poorly Water-Soluble Drugs: The Industrial Perspective. Expert Opin. Drug Deliv. 2011, 8, 1481–1500. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O., III; Watts, A.B.; Miller, D.A. Formulating Poorly Water Soluble Drugs, 2nd ed.; Springer: Berlin, Germany, 2016; Volume 22. [Google Scholar]
- Chiou, W.L.; Riegelman, S. Pharmaceutical Applications of Solid Dispersion Systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.P.; Dugar, R.P. Application of Surfactants in Solid Dispersion Technology for Improving Solubility of Poorly Water Soluble Drugs. J. Drug Deliv. Sci. Technol. 2017, 41, 68–77. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. Characteristics and Significance of the Amorphous State in Pharmaceutical Systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.; Kumar, S.; Zhou, Q. Pharmaceutical Amorphous Solid Dispersion: A Review of Manufacturing Strategies. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Singh, A.; Van Den Mooter, G. Spray Drying Formulation of Amorphous Solid Dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing Facts Behind Spray Dried Solid Dispersion Technology Used for Solubility Enhancement. Saudi Pharm. J. 2015, 23, 352–365. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, T.; Marques, S.; das Neves, J.; Sarmento, B. Amorphous Solid Dispersions: Rational Selection of a Manufacturing Process. Adv. Drug Deliv. Rev. 2016, 100, 85–101. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering Via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, K.; Niwa, T.; Danjo, K. Preparation of Sustained-Release Coated Particles by Novel Microencapsulation Method Using Three-Fluid Nozzle Spray Drying Technique. Eur. J. Pharm. Sci. 2014, 51, 11–19. [Google Scholar] [CrossRef]
- Sunderland, T.; Kelly, J.G.; Ramtoola, Z. Application of a Novel 3-Fluid Nozzle Spray Drying Process for the Microencapsulation of Therapeutic Agents Using Incompatible Drug-Polymer Solutions. Arch. Pharmacal Res. 2015, 38, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Tagawa, M.; Hoshi, N.; Ogura, T.; Okamoto, H.; Danjo, K. Improved Dissolution of an Insoluble Drug Using A 4-Fluid Nozzle Spray-Drying Technique. Chem. Pharm. Bull. 2004, 52, 1066–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumondor, A.C.; Marsac, P.J.; Stanford, L.A.; Taylor, L.S. Phase Behavior of Poly (Vinylpyrrolidone) Containing Amorphous Solid Dispersions in the Presence of Moisture. Mol. Pharm. 2009, 6, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.T.; Blaabjerg, L.I.; Lenz, E.; Bohr, A.; Grohganz, H.; Kleinebudde, P.; Rades, T.; Löbmann, K. Preparation and Characterization of Spray-Dried Co-Amorphous Drug-Amino Acid Salts. J. Pharm. Pharmacol. 2016, 68, 615–624. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Zemlyanov, D.Y.; Cavallaro, A.; Mangal, S.; Taylor, L.S.; Zhou, Q.T. Qualitative and Quantitative Characterization of Composition Heterogeneity on the Surface of Spray Dried Amorphous Solid Dispersion Particles by an Advanced Surface Analysis Platform with High Surface Sensitivity and Superior Spatial Resolution. Mol. Pharm. 2018, 15, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.V.; Pathak, V.; Zemlyanov, D.Y.; Taylor, L.S.; Zhou, Q.T. Physical Stability and Dissolution of Lumefantrine Amorphous Solid Dispersions Produced by Spray Anti-Solvent Precipitation. J. Pharm. Sci. 2021, 110, 2423–2431. [Google Scholar] [CrossRef]
- Trasi, N.S.; Bhujbal, S.V.; Zemlyanov, D.Y.; Zhou, Q.T.; Taylor, L.S. Physical Stability and Release Properties of Lumefantrine Amorphous Solid Dispersion Granules Prepared by a Simple Solvent Evaporation Approach. Int. J. Pharm. X 2020, 2, 100052. [Google Scholar] [CrossRef]
- Li, M.; Koranne, S.; Fang, R.; Lu, X.; Williams, D.M.; Munson, E.J.; Bhambhani, A.; Su, Y. Probing Microenvironmental Acidity in Lyophilized Protein and Vaccine Formulations Using Solid-state NMR Spectroscopy. J. Pharm. Sci. 2021, 110, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Purohit, H.S.; Ormes, J.D.; Saboo, S.; Su, Y.; Lamm, M.S.; Mann, A.K.; Taylor, L.S. Insights into Nano-And Micron-Scale Phase Separation in Amorphous Solid Dispersions Using Fluorescence-Based Techniques in Combination with Solid State Nuclear Magnetic Resonance Spectroscopy. Pharm. Res. 2017, 34, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.; Zografi, G. Water Vapor Absorption into Amorphous Hydrophobic Drug/Poly (Vinylpyrrolidone) Dispersions. J. Pharm. Sci. 2002, 91, 2150. [Google Scholar] [CrossRef] [PubMed]
- Rumondor, A.C.; Taylor, L.S. Application of Partial Least-Squares (PLS) Modeling in Quantifying Drug Crystallinity in Amorphous Solid Dispersions. Int. J. Pharm. 2010, 398, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, S.; Lowinger, M.B.; Liu, X.; Lu, X.; Su, Y.; Williams, R.O., III. Influence of Mechanical and Thermal Energy on Nifedipine Amorphous Solid Dispersions Prepared by Hot Melt Extrusion: Preparation and Physical Stability. Int. J. Pharm. 2019, 561, 324–334. [Google Scholar] [CrossRef]
- Brettmann, B.; Bell, E.; Myerson, A.; Trout, B. Solid-State NMR Characterization of High-Loading Solid Solutions of API and Excipients Formed by Electrospinning. J. Pharm. Sci. 2012, 101, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Sarpal, K.; Tower, C.W.; Munson, E.J. Investigation into Intermolecular Interactions and Phase Behavior of Binary and Ternary Amorphous Solid Dispersions of Ketoconazole. Mol. Pharm. 2019, 17, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Sperger, D.; Munson, E.J. Investigating Miscibility and Molecular Mobility of Nifedipine-PVP Amorphous Solid Dispersions Using Solid-State NMR Spectroscopy. Mol. Pharm. 2014, 11, 329–337. [Google Scholar] [CrossRef]
- Lu, X.; Huang, C.; Lowinger, M.B.; Yang, F.; Xu, W.; Brown, C.D.; Hesk, D.; Koynov, A.; Schenck, L.; Su, Y. Molecular Interactions in Posaconazole Amorphous Solid Dispersions from Two-Dimensional Solid-State NMR Spectroscopy. Mol. Pharm. 2019, 16, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Rumondor, A.C.; Stanford, L.A.; Taylor, L.S. Effects of Polymer Type and Storage Relative Humidity on the Kinetics of Felodipine Crystallization from Amorphous Solid Dispersions. Pharm. Res. 2009, 26, 2599. [Google Scholar] [CrossRef]
- Qian, F.; Huang, J.; Zhu, Q.; Haddadin, R.; Gawel, J.; Garmise, R.; Hussain, M. Is a Distinctive Single Tg a Reliable Indicator for the Homogeneity of Amorphous Solid Dispersion? Int. J. Pharm. 2010, 395, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle Formation in Spray Drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Gengenbach, T.; Denman, J.A.; Heidi, H.Y.; Li, J.; Chan, H.K. Synergistic Antibiotic Combination Powders of Colistin and Rifampicin Provide High Aerosolization Efficiency and Moisture Protection. AAPS J. 2014, 16, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.T.; Loh, Z.H.; Yu, J.; Sun, S.P.; Gengenbach, T.; Denman, J.A.; Li, J.; Chan, H.-K. How Much Surface Coating of Hydrophobic Azithromycin is Sufficient to Prevent Moisture-Induced Decrease in Aerosolisation of Hygroscopic Amorphous Colistin Powder? AAPS J. 2016, 18, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, Q.T.; Sun, S.-P.; Denman, J.A.; Gengenbach, T.R.; Barraud, N.; Rice, S.A.; Li, J.; Yang, M.; Chan, H.-K. Effects of Surface Composition on the Aerosolisation and Dissolution of Inhaled Antibiotic Combination Powders Consisting of Colistin And Rifampicin. AAPS J. 2016, 18, 372–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Formulation | Theoretical Surface Composition (% Weight) | Practical Surface Composition (% Weight) | ||
|---|---|---|---|---|
| Naproxen | PVP | Naproxen | PVP | |
| 2FN | 20 | 80 | 20 ± 1 | 80 ± 1 |
| 3FN | 20 | 80 | 17± 2 | 82 ± 2 |
| Formulation | T1 (s) | Homogeneous | Domain Size (nm) | T1rho (ms) | Miscible | Domain Size (nm) | |
|---|---|---|---|---|---|---|---|
| 2FN | Naproxen | 2.58 ± 0.09 | Yes | 112 | 17 ± 2 | Yes | 9 |
| PVP | 2.64 ± 0.04 | 18 ± 3 | |||||
| 3FN | Naproxen | 2.97 ± 0.43 | Yes | 120 | 18 ± 2 | No | 9 |
| PVP | 3.05 ± 0.09 | 27 ± 1 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhujbal, S.V.; Su, Y.; Pathak, V.; Zemlyanov, D.Y.; Cavallaro, A.-A.; Munson, E.J.; Taylor, L.S.; Zhou, Q. Effect of Storage Humidity on Physical Stability of Spray-Dried Naproxen Amorphous Solid Dispersions with Polyvinylpyrrolidone: Two Fluid Nozzle vs. Three Fluid Nozzle. Pharmaceutics 2021, 13, 1074. https://doi.org/10.3390/pharmaceutics13071074
Bhujbal SV, Su Y, Pathak V, Zemlyanov DY, Cavallaro A-A, Munson EJ, Taylor LS, Zhou Q. Effect of Storage Humidity on Physical Stability of Spray-Dried Naproxen Amorphous Solid Dispersions with Polyvinylpyrrolidone: Two Fluid Nozzle vs. Three Fluid Nozzle. Pharmaceutics. 2021; 13(7):1074. https://doi.org/10.3390/pharmaceutics13071074
Chicago/Turabian StyleBhujbal, Sonal V., Yongchao Su, Vaibhav Pathak, Dmitry Y. Zemlyanov, Alex-Anthony Cavallaro, Eric J. Munson, Lynne S. Taylor, and Qi (Tony) Zhou. 2021. "Effect of Storage Humidity on Physical Stability of Spray-Dried Naproxen Amorphous Solid Dispersions with Polyvinylpyrrolidone: Two Fluid Nozzle vs. Three Fluid Nozzle" Pharmaceutics 13, no. 7: 1074. https://doi.org/10.3390/pharmaceutics13071074