Effect of Carbon Chain Length, Ionic Strength, and pH on the In Vitro Release Kinetics of Cationic Drugs from Fatty-Acid-Loaded Contact Lenses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fatty-Acid-Loading into Pristine Silicone Hydrogel Contact Lenses
2.3. Phytosphingosine-Loading into Pristine Silicone Hydrogel Contact Lenses
2.4. Drug-Loading into Fatty-Acid-Loaded Lenses or Phytosphingosine-Loaded Lenses
2.5. Drug Release Experiments
2.5.1. Effect of Ionic Strength in Release Medium on Drug Release Kinetics
2.5.2. Effect of pH of Release Medium on Drug Release Kinetics
3. Results and Discussion
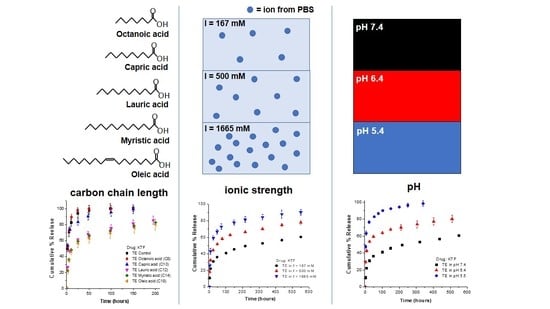
3.1. Effect of Fatty Acid Carbon Chain Length on Release Kinetics of Cationic Drugs
3.2. Effect of Ionic Strength of Release Medium on Release Kinetics
3.3. Effect of pH of Release Medium on Release Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourlais, C.L.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic Drug Delivery Systems--Recent Advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Hui, A. Contact Lenses for Ophthalmic Drug Delivery. Clin. Exp. Optom. 2017, 100, 494–512. [Google Scholar] [CrossRef] [Green Version]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-Based Ocular Drug Delivery Systems for Hydrophobic Drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A Comprehensive Review on Contact Lens for Ophthalmic Drug Delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Hoare, T.R.; Iwata, N.G.; Behlau, I.; Dohlman, C.H.; Langer, R.; Kohane, D.S. A Drug-Eluting Contact Lens. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3346. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Wang, N.; Hu, N. Nanostructured-Based Soft Contact Lenses for Controlled Delivery of Ophthalmic Drugs. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5384. [Google Scholar]
- Paugh, J.; Stapleton, F.; Keay, L.; Ho, A. Tear Exchange under Hydrogel Contact Lenses: Methodological Considerations. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2813–2820. [Google Scholar]
- Hehl, E.; Beck, R.; Luthard, K.; Guthoff, R.; Drewelow, B. Improved Penetration of Aminoglycosides and Fluorozuinolones into the Aqueous Humour of Patients by Means of Acuvue Contact Lenses. Eur. J. Clin. Pharmacol. 1999, 55, 317–323. [Google Scholar] [CrossRef]
- González-Chomón, C.; Concheiro, A.; Alvarez-Lorenzo, C. Soft Contact Lenses for Controlled Ocular Delivery: 50 Years in the Making. Ther. Deliv. 2013, 4, 1141–1161. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Chauhan, A. Ophthalmic Drug Delivery by Contact Lenses. Expert Rev. Ophthalmol. 2012, 7, 199–201. [Google Scholar] [CrossRef]
- Fan, X.; Torres-Luna, C.; Azadi, M.; Domszy, R.; Hu, N.; Yang, A.; David, A.E. Evaluation of Commercial Soft Contact Lenses for Ocular Drug Delivery: A Review. Acta Biomater. 2020, 115, 60–74. [Google Scholar] [CrossRef]
- Lanier, O.L.; Christopher, K.G.; Macoon, R.M.; Yu, Y.; Sekar, P.; Chauhan, A. Commercialization Challenges for Drug Eluting Contact Lenses. Expert Opin. Drug Deliv. 2020, 17, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Dispersion of Microemulsion Drops in HEMA Hydrogel: A Potential Ophthalmic Drug Delivery Vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Chauhan, A. Ophthalmic Delivery of Cyclosporine A from Brij-97 Microemulsion and Surfactant-Laden p-HEMA Hydrogels. Int. J. Pharm. 2008, 361, 222–229. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Koolivand, A.; Fan, X.; Zhu, Y.; Domszy, R.; Yang, J.; Yang, A.; Wang, N.S. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics 2019, 11, 262. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.-C.; Kim, J.; Chauhan, A. Extended Delivery of Hydrophilic Drugs from Silicone-Hydrogel Contact Lenses Containing Vitamin E Diffusion Barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef]
- Sekar, P.; Chauhan, A. Effect of Vitamin-E Integration on Delivery of Prostaglandin Analogs from Therapeutic Lenses. J. Colloid Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended Delivery of Non-Steroidal Anti-Inflammatory Drugs through Contact Lenses Loaded with Vitamin E and Cationic Surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-Laden Soft Contact Lenses for Extended Delivery of Ophthalmic Drugs. Biomaterials 2009, 30, 867–878. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Koolivand, A.; Fan, X.; Agrawal, N.R.; Hu, N.; Zhu, Y.; Domszy, R.; Briber, R.M.; Wang, N.S.; Yang, A. Formation of Drug-Participating Catanionic Aggregates for Extended Delivery of Non-Steroidal Anti-Inflammatory Drugs from Contact Lenses. Biomolecules 2019, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Gulsen, D.; Li, C.-C.; Chauhan, A. Dispersion of DMPC Liposomes in Contact Lenses for Ophthalmic Drug Delivery. Curr. Eye Res. 2005, 30, 1071–1080. [Google Scholar] [CrossRef]
- Danion, A.; Brochu, H.; Martin, Y.; Vermette, P. Fabrication and Characterization of Contact Lenses Bearing Surface-Immobilized Layers of Intact Liposomes. J. Biomed. Mater. Res. A 2007, 82, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, H.; Alvarez-Lorenzo, C. Timolol Uptake and Release by Imprinted Soft Contact Lenses Made of N,N-Diethylacrylamide and Methacrylic Acid. J. Control. Release 2002, 83, 223–230. [Google Scholar] [CrossRef]
- Bengani, L.C.; Chauhan, A. Extended Delivery of an Anionic Drug by Contact Lens Loaded with a Cationic Surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef]
- Zhang, X.; Xiuzhen, C.; Ping, Q. Therapeutic Contact Lenses for Ophthalmic Drug Delivery: Major Challenges. J. Biomater. Sci. Polym. Ed. 2020, 31, 549–560. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Fan, X.; Domszy, R.; Yang, J.; Briber, R.M.; Yang, A. Extended Delivery of Cationic Drugs from Contact Lenses Loaded with Unsaturated Fatty Acids. Eur. J. Pharm. Biopharm. 2020, 155, 1–11. [Google Scholar] [CrossRef]
- Stryer, L.; Berg, J.; Tymoczko, J. Biochemistry, 5th ed.; WH Freeman & Co Ltd: New York, NY, USA, 2002. [Google Scholar]
- Kim, J.; Chauhan, A. Dexamethasone Transport and Ocular Delivery from Poly(Hydroxyethyl Methacrylate) Gels. Int. J. Pharm. 2007, 353, 205–222. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and Medium-Chain Fatty Acids Exhibit Antimicrobial Activity for Oral Microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knothe, G.; Dunn, R.O. A Comprehensive Evaluation of the Melting Points of Fatty Acids and Esters Determined by Differential Scanning Calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Quast, K. Flotation of Hematite Using C6–C18 Saturated Fatty Acids. Miner. Eng. 2006, 19, 582–597. [Google Scholar] [CrossRef]
- Dev Kumar, G.; Micallef, S.A. Susceptibility of Salmonella Enterica Isolates from Tomato Farm Environments to Fatty Acids Naturally Found on Tomato Fruit. Foodborne Pathog. Dis. 2017, 14, 293–301. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. In Vitro and in Vivo Evaluation of Ketotifen Fumarate-Loaded Silicone Hydrogel Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2011, 18, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Pall, B.; Gomes, P.; Yi, F.; Torkildsen, G. Management of Ocular Allergy Itch With an Antihistamine-Releasing Contact Lens. Cornea 2019, 38, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-C.; Burke, M.T.; Chauhan, A. Transport of Topical Anesthetics in Vitamin E Loaded Silicone Hydrogel Contact Lenses. Langmuir 2012, 28, 1478–1487. [Google Scholar] [CrossRef]
- Pang, X.; Fan, T. Cytotoxic Effect and Possible Mechanisms of Tetracaine on Human Corneal Epithelial Cells in Vitro. Int. J. Ophthalmol. 2016, 9, 497. [Google Scholar]
- Pimenta, A.F.R.; Ascenso, J.; Fernandes, J.C.S.; Colaço, R.; Serro, A.P.; Saramago, B. Controlled Drug Release from Hydrogels for Contact Lenses: Drug Partitioning and Diffusion. Int. J. Pharm. 2016, 515, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Mao, S. Enhanced Drug Loading Efficiency of Contact Lenses via Salt-Induced Modulation. Asian J. Pharm. Sci. 2019, 14, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.-P.-D.; Yang, M.-C. Synthesis and Characterization of Silicone Contact Lenses Based on TRIS-DMA-NVP-HEMA Hydrogels. Polymers 2019, 11, 944. [Google Scholar] [CrossRef] [Green Version]
- Dixon, P.; Chauhan, A. Effect of the Surface Layer on Drug Release from Delefilcon-A (Dailies Total1®) Contact Lenses. Int. J. Pharm. 2017, 529, 89–101. [Google Scholar] [CrossRef]
- Dursch, T.J.; Liu, D.E.; Oh, Y.; Radke, C.J. Fluorescent Solute-Partitioning Characterization of Layered Soft Contact Lenses. Acta Biomater. 2015, 15, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Stone, R. Introducing Water Gradient Technology. Contact Lens Spectr. 2013, 28, 34–38. [Google Scholar]
- Zhu, Q.; Liu, C.; Sun, Z.; Zhang, X.; Liang, N.; Mao, S. Inner Layer-Embedded Contact Lenses for pH-Triggered Controlled Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2018, 128, 220–229. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, K.-S.; Kim, J.-W.; Kang, J.-Y.; Kim, J.-K. Stimulus-Responsive Contact Lens for IOP Measurement or Temperature-Triggered Drug Release. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Maulvi, F.A.; Choksi, H.H.; Desai, A.R.; Patel, A.S.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. pH Triggered Controlled Drug Delivery from Contact Lenses: Addressing the Challenges of Drug Leaching during Sterilization and Storage. Colloids Surf. B Biointerfaces 2017, 157, 72–82. [Google Scholar] [CrossRef]
- Kim, H.-J.; Zhang, K.; Moore, L.; Ho, D. Diamond Nanogel-Embedded Contact Lenses Mediate Lysozyme-Dependent Therapeutic Release. ACS Nano 2014, 8, 2998–3005. [Google Scholar] [CrossRef]
- Lee, D.; Lee, N.; Kwon, I. Efficient Loading of Ophthalmic Drugs with Poor Loadability into Contact Lenses Using Functional Comonomers. Biomater. Sci. 2018, 6, 2639–2646. [Google Scholar] [CrossRef]
- Contreras, F.-X.; Sot, J.; Alonso, A.; Goñi, F.M. Sphingosine Increases the Permeability of Model and Cell Membranes. Biophys. J. 2006, 90, 4085–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, P.; Sallmann, A.; Wiesenberg, I. Synthesis and Quantitative Structure-Activity Relationships of Diclofenac Analogs. J. Med. Chem. 1990, 33, 2358–2368. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Hiratani, H.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Soft Contact Lenses Capable of Sustained Delivery of Timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing Targeted Antibiotic Therapy via pH Responsive Solid Lipid Nanoparticles from an Acid Cleavable Lipid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.; Omolo, C.A.; Gannimani, R.; Waddad, A.Y.; Rambharose, S.; Mocktar, C.; Singh, S.; Parboosing, R.; Govender, T. Novel Fatty Acid-Based pH-Responsive Nanostructured Lipid Carriers for Enhancing Antibacterial Delivery. J. Drug Deliv. Sci. Technol. 2019, 53, 101125. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Chen, H.-H.; Huang, W.-C.; Chiang, W.-H.; Liu, T.-I.; Shen, M.-Y.; Hsu, Y.-H.; Lin, S.-C. pH-Responsive Therapeutic Solid Lipid Nanoparticles for Reducing P-Glycoprotein-Mediated Drug Efflux of Multidrug Resistant Cancer Cells. Int. J. Nanomed. 2015, 10, 5035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhamis, K.A.; Salem, M.S.; Khanfar, M.S. The Sorption of Ketotifen Fumarate by Chitosan. AAPS PharmSciTech 2008, 9, 866–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Commercial Name | Material | Manufacturer | Water Content (%) | Modulus (MPa) | Center Thickness (mm) |
|---|---|---|---|---|---|
| ACUVUE TruEye® | Narafilcon A | Johnson & Johnson Vision Care | 46% | 0.66 | 0.09 |
| Dailies Total1® | Delefilcon A | Alcon Inc. | 33% (bulk) ≥80% (surface) | 0.7 | 0.09 |
| Fatty Acid | # of Carbons and Degree of Saturation | Melting Point a (C) | Solubility in Water b (M) |
|---|---|---|---|
| Octanoic acid | 8:0 | 16–17 | 4.7 × 10−3 |
| Capric acid | 10:0 | 31–32 | 3.0 × 10−4 |
| Lauric acid | 12:0 | 43–45 | 1.2 × 10−5 |
| Myristic acid | 14:0 | 53–58 | 1.0 × 10−6 |
| Oleic acid | 18:1; (cis)9 | 10–16 | 4.1 × 10−8 c |
| Contact Lens | Fatty Acid Loaded | Tetracaine Hydrochloride | Ketotifen Fumarate | ||
|---|---|---|---|---|---|
| Amount of Drug Uptake (µg/Lens) | Time for 70% Cumulative Drug Release (h) | Amount of Drug Uptake (µg/Lens) | Time for 70% Cumulative Drug Release (h) | ||
| ACUVUE TruEye | Control | 120.3 ± 4.0 | 3.34 ± 0.21 | 211.1 ± 13.0 | 5.41 ± 1.35 |
| C8 | 46.2 ± 3.6 | 1.47 ± 0.14 | 117.9. ± 11.0 | 4.62 ± 0.40 | |
| C10 | 140.9 ± 3.1 | 2.76 ± 0.79 | 237.7 ± 12.0 | 6.10 ± 0.74 | |
| C12 | 356.3 ± 10.6 | 27.5 ± 3.56 | 540 ± 21.3 | 62.9 ± 5.66 | |
| C14 | 349.2 ± 15.0 | 45.4 ± 3.73 | 516.4 ± 4.1 | 79.4 ± 3.55 | |
| C18 | 330.6 ± 17.8 | 25.1 ± 1.58 | 502.3 ± 39.2 | 101.8 ± 41.4 | |
| Dailies Total | Control | 99.4 ±1.7 | 1.86 ± 0.13 | 152.8 ± 7.1 | 2.37 ± 0.48 |
| C8 | 47.7 ± 5.0 | 1.73 ± 0.05 | 92.3. ± 13.2 | 2.60 ± 0.11 | |
| C10 | 104.4 ± 12.3 | 1.79 ± 0.15 | 172.3 ± 3.0 | 1.95 ± 0.04 | |
| C12 | 255.0 ± 15.3 | 9.90 ± 1.09 | 390.9 ± 26.0 | 24.7 ± 3.54 | |
| C14 | 268.7 ± 11.1 | 20.0 ± 5.68 | 339.5 ± 15.9 | 22.3 ± 2.89 | |
| C18 | 230.6 ± 24.5 | 11.1 ± 6.31 | 321.0 ± 23.9 | 21.7 ± 6.60 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Luna, C.; Hu, N.; Domszy, R.; Fan, X.; Yang, J.; Briber, R.M.; Wang, N.S.; Yang, A. Effect of Carbon Chain Length, Ionic Strength, and pH on the In Vitro Release Kinetics of Cationic Drugs from Fatty-Acid-Loaded Contact Lenses. Pharmaceutics 2021, 13, 1060. https://doi.org/10.3390/pharmaceutics13071060
Torres-Luna C, Hu N, Domszy R, Fan X, Yang J, Briber RM, Wang NS, Yang A. Effect of Carbon Chain Length, Ionic Strength, and pH on the In Vitro Release Kinetics of Cationic Drugs from Fatty-Acid-Loaded Contact Lenses. Pharmaceutics. 2021; 13(7):1060. https://doi.org/10.3390/pharmaceutics13071060
Chicago/Turabian StyleTorres-Luna, Cesar, Naiping Hu, Roman Domszy, Xin Fan, Jeff Yang, Robert M. Briber, Nam Sun Wang, and Arthur Yang. 2021. "Effect of Carbon Chain Length, Ionic Strength, and pH on the In Vitro Release Kinetics of Cationic Drugs from Fatty-Acid-Loaded Contact Lenses" Pharmaceutics 13, no. 7: 1060. https://doi.org/10.3390/pharmaceutics13071060