Nanoencapsulated Doxorubicin Prevents Mucositis Development in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NLC–DOX and Free DOX Solution
2.3. Particle Size Analysis and Zeta Potential
2.4. Nanoparticle Tracking Analysis (NTA)
2.5. Drug Encapsulation Efficiency (EE) and Drug Loading (DL)
2.6. Differential Scanning Calorimetry
2.7. ATR–FTIR Analysis
2.8. Transmission Electron Microscopy (TEM) and Cryogenic TEM (Cryo-TEM)
2.9. In Vitro DOX Release
2.10. Animals and Experimental Groups
2.11. Histological Analysis
2.12. Analysis of Gene Expression
2.13. Assessment of Intestinal Permeability
2.14. Statistical Analysis
3. Results
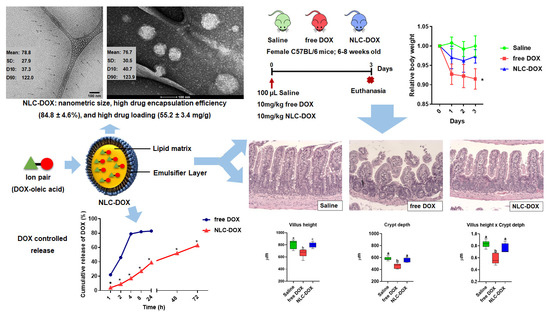
3.1. Characterization of the Developed NLC-DOX
3.2. In Vitro DOX Release
3.3. NLC-DOX Treatment Prevents Weight Loss in C57BL/6 Mice
3.4. NLC-DOX Preserves Bowel Architecture, Prevents Mucositis-Compatible Ulcerations and Improves Intestinal Permeability
3.5. NLC-DOX Formulation Maintains Cytokine Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020.
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces Sci. 2011, 85, 262–269. [Google Scholar] [CrossRef]
- Fernandes, E.; Ferreira, D.; Peixoto, A.; Freitas, R.; Relvas-Santos, M.; Palmeira, C.; Martins, G.; Barros, A.; Santos, L.L.; Sarmento, B.; et al. Glycoengineered nanoparticles enhance the delivery of 5-fluoroucil and paclitaxel to gastric cancer cells of high metastatic potential. Int. J. Pharm. 2019, 30, 570. [Google Scholar] [CrossRef]
- Awasthi, R.; Roseblade, A.; Hansbro, P.M.; Rathbone, M.J.; Dua, K.; Bebawy, M. Nanoparticles in Cancer Treatment: Opportunities and Obstacles. Curr. Drug Targets 2018, 19, 1696–1709. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003, 98, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Pulito, C.; Cristaudo, A.; La Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of câncer therapy. J. Exp. Clin. Cancer Res. 2020, 210. [Google Scholar] [CrossRef]
- Lai, H.C.; Yeh, Y.C.; Ting, C.T.; Lee, W.L.; Lee, H.W.; Wang, L.C.; Wang, K.Y.; Lai, H.C.; Wu, A.; Liu, T.J. Doxycycline suppresses doxorubicin-induced oxidative stress and cellular apoptosis in mouse hearts. Eur. J. Pharmacol. 2010, 644, 176–187. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Anthracycline Nano-Delivery Systems to Overcome Multiple Drug Resistance: A Comprehensive Review. Nano Today 2013, 8, 313–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaikomon, K.; Chattong, S.; Chaiya, T.; Tiwawech, D.; Sritana-Anant, Y.; Sereemaspun, A.; Manotham, K. Doxorubicin-conjugated dexamethasone induced MCF-7 apoptosis without entering the nucleus and able to overcome MDR-1-induced resistance. Drug Des. Dev. Ther. 2018, 12, 2361–2369. [Google Scholar] [CrossRef] [Green Version]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Goto, S.; Ihara, Y.; Urata, Y.; Izumi, S.; Abe, K.; Koji, T.; Kondo, T. Doxorubicin-induced DNA intercalation and scavenging by nuclear glutathione S-transferase π. FASEB J. 2001, 15, 2702–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodley, A.; Liu, L.F.; Israel, M.; Seshadri, R.; Koseki, Y.; Giuliani, F.C.; Kirschenbaum, S.; Silber, R.; Potmesil, M. DNA Topoisomerase II-mediated Interaction of Doxorubicin and Daunorubicin Congeners with DNA. Cancer Res. 1989, 49, 5969–5978. [Google Scholar]
- Asensio-López, M.C.; Soler, F.; Sánchez-Más, J.; Pascual-Figal, D.; Fernández-Belda, F.; Lax, A. Early oxidative damage induced by doxorubicin: Source of production, protection by GKT137831 and effect on Ca2+ transporters in HL-1 cardiomyocytes. Arch. Biochem. Biophys. 2016, 594, 26–36. [Google Scholar] [CrossRef]
- McCullough, R.W. US oncology-wide incidence, duration, costs and deaths from chemoradiation mucositis and antimucositis therapy benefits. Future Oncol. 2017, 13, 2823–2852. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Cochran, T.R.; Franco, V.I.; Miller, T.L. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. 2013, 10, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Moon, M.; Dawood, S.; McManus, B.; Liu, P.P. Mechanisms and management of doxorubicin cardiotoxicity. Herz 2011, 36, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Raber-Durlacher, J.E.; Weijl, N.I.; Abu Saris, M.; de Koning, B.; Zwinderman, A.H.; Osanto, S. Oral mucositis in patients treated with chemotherapy for solid tumors: A retrospective analysis of 150 cases. Support Care Cancer 2000, 8, 366–371. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Brinkman, B.M.; Heyndrickx, L.; Vandenabeele, P.; Krysko, D.V. Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J. Pathol. 2012, 226, 598–608. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Florczak, A.; Deptuch, T.; Penderecka, K.; Jastrzebska, K.; Mackiewicz, A.; Dams-Kozlowska, H. Drug affinity and targeted delivery: Double functionalization of silk spheres for controlled doxorubicin delivery into Her2-positive cancer cells. J. Nanobiotechnol. 2020, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Liu, P. Doxorubicin-doxorubicin conjugate prodrug as drug self-delivery system for intracellular pH-riggered slow release. Colloids Surf. B Biointerfaces 2020, 1, 185. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Nkambule, T.T.I.; Mamba, B.B.; Msagati, T.A.M. The stimuli-responsive properties of doxorubicin adsorbed onto bimetallic Au@Pd nanodendrites and its potential application as drug delivery platform. Mater. Sci. Eng. C 2020, 110, 110696. [Google Scholar] [CrossRef]
- Wang, C.; Qi, P.; Lu, Y.; Liu, L.; Zhang, Y.; Sheng, Q.; Wang, T.; Zhang, M.; Wang, R.; Song, S. Bicomponent polymeric micelles for pH-controlled delivery of doxorubicin. Drug Deliv. 2020, 27, 344–357. [Google Scholar] [CrossRef]
- Sedlacek, O.; Driessche, A.V.; Uvyn, A.; Geest, B.G.D.; Hoogenboom, R. Poly(2-methyl-2-oxazoline) conjugates with doxorubicin: From synthesis of high drug loading water-soluble constructs to in vitro anti-cancer properties. J. Control. Release 2020, 326, 53–62. [Google Scholar] [CrossRef]
- Fraix, A.; Conte, C.; Gazzano, E.; Riganti, C.; Quaglia, F.; Sortino, S. Overcoming Doxorubicin Resistance with Lipid-Polymer Hybrid Nanoparticles Photoreleasing Nitric Oxide. Mol. Pharm. 2020, 17, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Mussi, S.V.; Sawant, R.; Perche, F.; Oliveira, M.C.; Azevedo, R.B.; Ferreira, L.A.M.; Torchilin, V.P. Novel Nanostructured Lipid Carrier Co-Loaded with Doxorubicin and Docosahexaenoic Acid Demonstrates Enhanced in Vitro Activity and Overcomes Drug Resistance in MCF-7/Adr Cells. Pharm. Res. 2014, 31, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Minh, L.V.; Li, N.; Garamus, V.M.; Handge, U.A.; Liu, J.; Zou, A. Doxorubicin hydrochloride-oleic acid conjugate loaded nanostructured lipid carriers for tumor specific drug release. Colloids Surf. B Biointerfaces 2016, 145, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Popilski, H.; Feinshtein, V.; Kleiman, S.; Mattarei, A.; Garofalo, M.; Salmaso, S.; Stepensky, D. Doxorubicin liposomes cell penetration enhancement and its potential drawbacks for the tumor targeting efficiency. Int. J. Pharm. 2021, 592, 120012. [Google Scholar] [CrossRef] [PubMed]
- Makwana, V.; Karanjia, J.; Haselhorst, T.; Anoopkumar-Dukie, S.; Rudrawar, S. Liposomal doxorubicin as targeted delivery platform: Current trends in surface functionalization. Int. J. Pharm. 2021, 593, 120117. [Google Scholar] [CrossRef]
- Perez, A.T.; Domenech, G.H.; Frankel, C.; Vogel, C.L. Pegylated liposomal doxorubicin (Doxil) for metastatic breast cancer: The Cancer Research Network, Inc., experience. Cancer Investig. 2002, 20, 22–29. [Google Scholar] [CrossRef]
- Leonard, R.C.F.; Williams, S.; Tulpule, A.; Levine, A.M.; Oliveros, S. Improving the therapeutic index of anthracycline chemotherapy: Focus on liposomal doxorubicin (Myocet™). Breast 2009, 18, 218–224. [Google Scholar] [CrossRef]
- Lages, E.B.; Fernandes, R.S.; Silva, J.O.; de Souza, Â.M.; Cassali, G.D.; de Barros, A.L.B.; Ferreira, L.A.M. Co-delivery of doxorubicin, docosahexaenoic acid, and α-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2020, 132, 110876. [Google Scholar] [CrossRef] [PubMed]
- Dingler, A.; Gohla, S. Production of solid lipid nanoparticles (SLN): Scaling up feasibilities. J. Microencapsul. 2002, 19, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Holm, R. Solid lipid nanocarriers in drug delivery: Characterization and design. Expert Opin Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shidhaye, S.S.; Vaidya, R.; Sutar, S.; Patwardhan, A.; Kadam, V.J. Solid lipid nanoparticles and nanostructured lipid carriers--innovative generations of solid lipid carriers. Curr. Drug Deliv. 2008, 5, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 131–155. [Google Scholar] [CrossRef]
- Wu, M.; Fan, Y.; Lv, S.; Xiao, B.; Ye, M.; Zhu, X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: A comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016, 23, 2720–2725. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Zhu, J.; Gao, Z. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int. J. Nanomed. 2018, 13, 303. [Google Scholar] [CrossRef] [Green Version]
- Mussi, S.V.; Silva, R.C.; Oliveira, M.C.; Lucci, C.M.; Azevedo, R.B.; Ferreira, L.A. New approach to improve encapsulation and antitumor activity of doxorubicin loaded in solid lipid nanoparticles. Eur. J. Pharm. Sci. 2013, 23, 282–290. [Google Scholar] [CrossRef]
- Fulop, Z.; Gref, R.; Loftsson, T. A permeation method for detection of self-aggregation of doxorubicin in aqueous environment. Int. J. Pharm. 2013, 15, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Peira, E.; Chirio, D.; Muntoni, E.; Biasibetti, E.; Capucchio, M.T.; Valazza, A.; Panciani, P.P.; Lanotte, M.; et al. Solid lipid nanoparticles for potential doxorubicin delivery in glioblastoma treatment: Preliminary in vitro studies. J. Pharm. Sci. 2014, 103, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Dong, X.; Swadley, C.L.; Gupte, A.; Leggas, M.; Ledebur, H.C.; Mumper, R.J. Development of idarubicin and doxorubicin solid lipid nanoparticles to overcome Pgp-mediated multiple drug resistance in leukemia. J. Biomed. Nanotechnol. 2009, 5, 151–161. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Mussi, S.V.; Gomes, D.A.; Yoshida, M.I.; Frezard, F.; Carregal, V.M.; Ferreira, L.A.M. α-Tocopherol Succinate Improves Encapsulation and Anticancer Activity of Doxorubicin Loaded in Solid Lipid Nanoparticles. Colloids Surf. B Biointerfaces Sci. 2016, 140, 246–253. [Google Scholar] [CrossRef]
- Subedi, R.K.; Kang, K.W.; Choi, H.K. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur. J. Pharm. Sci. 2009, 37, 508–513. [Google Scholar] [CrossRef]
- Miglietta, A.; Cavalli, R.; Bocca, C.; Ludovica, G.; Gasco, M.R. Cellular uptake and cytotoxicity of solid lipid nanospheres (SLN) incorporating doxorubicin or paclitaxel. Int. J. Pharm. 2000, 210, 61–67. [Google Scholar] [CrossRef]
- Zara, G.P.; Cavalli, R.; Bargoni, A.; Fundarò, A.; Vightto, D.; Gasco, M.R. Intravenous administration to rabbits of non-stealth and stealth doxorubicin-loaded solid lipid nanoparticles at increasing concentrations of stealth agent: Pharmacokinetics and distribution of doxorubicin in brain and other tissues. J. Drug Target 2002, 10, 327–335. [Google Scholar] [CrossRef]
- Steiniger, S.C.; Kreuter, J.; Khalansky, A.S.; Sckidan, I.N.; Bobruskin, A.I.; Smirnova, Z.S.; Severin, S.E.; UHL, R.; Kock, M.; Geiger, K.D.; et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int. J. Cancer 2004, 109, 759–767. [Google Scholar] [CrossRef]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Li, J.; Zhang, X.; Gong, T.; Zhang, Z. Lipid nanoemulsions loaded with doxorubicin-oleic acid ionic complex: Characterization, in vitro and in vivo studies. Pharmazie 2011, 66, 496–505. [Google Scholar] [PubMed]
- Siddiqui, A.; Gupta, V.; Liu, Y.Y.; Nazzal, S. Doxorubicin and MBO-asGCS oligonucleotide loaded lipid nanoparticles overcome multidrug resistance in adriamycin resistant ovarian cancer cells (NCI/ADR-RES). Int. J. Pharm. 2012, 431, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.H.; Huang, W.C.; Chiang, W.H.; Liu, T.I.; Shen, M.Y.; Hsu, Y.H.; Lin, S.C.; Chiu, H.C. pH-Responsive therapeutic solid lipid nanoparticles for reducing P-glycoprotein-mediated drug efflux of multidrug resistant cancer cells. Int. J. Nanomed. 2015, 10, 5035–5048. [Google Scholar] [CrossRef] [Green Version]
- Colas, S.; Maheo, K.; Denis, F.; Goupille, C.; Hoinard, C.; Champeroux, P.; Tranquart, F.; Bougnoux, P. Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: A role for tumor vascularization. Clin. Cancer Res. 2006, 19, 5879–5886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fundaro, A.; Cavalli, R.; Bargoni, A.; Vighetto, D.; Zara, G.P.; Gasco, M.R. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: Pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol. Res. 2000, 42, 337–343. [Google Scholar] [CrossRef]
- Borges, G.S.M.; Silva, J.O.; Fernandes, R.S.; de Souza, Â.M.; Cassali, G.D.; Yoshida, M.I.; Leite, E.A.; de Barros, A.L.B.; Ferreira, L.A.M. Sclareol is a potent enhancer of doxorubicin: Evaluation of the free combination and co-loaded nanostructured lipid carriers against breast cancer. Life Sci. 2019, 232, 116678. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Lu, T.; Ten Hagen, T.L.M. Inhomogeneous crystal grain formation in DPPC-DSPC based thermosensitive liposomes determines content release kinetics. J. Control. Release 2017, 247, 64–72. [Google Scholar] [CrossRef]
- Santos, R.G.C.; Viana, M.L.; Generoso, S.V.; Arantes, R.E.; Correia, M.I.T.D.; Cardoso, V.N. Glutamine supplementation decreases intestinal permeability and preserves gut mucosa integrity in an experimental mouse model. J. Parenter. Enter. Nutr. 2010, 34, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Rudra, A.; Deepa, R.M.; Ghosh, M.K.; Ghosh, S.; Mukherjee, B. Doxorubicin-loaded phosphatidylethanolamine-conjugated nanoliposomes: In Vitro characterization and their accumulation in liver, kidneys, and lungs in rats. Int. J. Nanomed. 2010, 5, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ma, Y.; Yue, X.; Cao, Z.; Dai, Z. One-pot construction of doxorubicin conjugated magnetic silica nanoparticles. New J. Chem. 2009, 33, 2414. [Google Scholar] [CrossRef]
- Shah, B.M.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: Risk assessment and QbD based optimization. J. Drug Deliv. Sci. Technol. 2016, 33, 37–50. [Google Scholar] [CrossRef]
- Kallakunta, V.R.; Tiwari, R.; Sarabu, S.; Bandari, S.; Repka, M.A. Effect of formulation and process variables on lipid based sustained release tablets via continuous twin screw granulation: A comparative study. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 121, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Pallerla, S.; Gauthier, T.; Sable, R.; Jois, S.D. Design of a doxorubicin-peptidomimetic conjugate that targets HER2-positive cancer cells. Eur. J. Med. Chem. 2017, 125, 914–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, R.S.; Silva, J.O.; Mussi, S.V.; Lopes, S.C.A.; Leite, E.A.; Cassali, G.D.; Cardoso, V.N.; Townsend, D.M.; Colletti, P.M.; Ferreira, L.A.M.; et al. Nanostructured Lipid Carrier Co-loaded with Doxorubicin and Docosahexaenoic Acid as a Theranostic Agent: Evaluation of Biodistribution and Antitumor Activity in Experimental Model. Mol. Imaging Biol. 2018, 20, 437–447. [Google Scholar] [CrossRef]
- Ni, S.; Qiu, L.; Zhang, G.; Zhou, H.; Han, Y. Lymph cancer chemotherapy: Delivery of doxorubicin-gemcitabine prodrug and vincristine by nanostructured lipid carriers. Int. J. Nanomed. 2017, 12, 1565–1576. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, C.B.; Parashar, P.; Arya, M. Biotin anchored nanostructured lipid carriers for targeted delivery of doxorubicin in management of mammary gland carcinoma through regulation of apoptotic modulator. J. Liposome Res. 2020, 30, 21–36. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hao, J.; Li, B.; Li, M.; Xiuwen, W. Lung cancer combination therapy: Co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2016, 23, 1398–1403. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Lima, B.H.S.; Goulart, G.A.C.; Mussi, S.V.; Borges, G.S.M.; Oréfice, R.L.; Ferreira, L.A.M. Improved Cytotoxic Effect of Doxorubicin by Its Combination with Sclareol in Solid Lipid Nanoparticle Suspension. J. Nanosci. Nanotechnol. 2018, 18, 5609–5616. [Google Scholar] [CrossRef]
- Singh, S.; Lohani, A.; Mishra, A.K.; Verma, A. Formulation and evaluation of carrot seed oil-based cosmetic emulsions. J. Cosmet. Laser Ther. 2018, 1–9. [Google Scholar] [CrossRef]
- Guilherme, V.A.; Ribeiro, L.N.M.; Alcântara, A.C.S.; Castro, S.R.; da Silva, G.H.R.; da Silva, C.G.; Breitkreitz, M.C.; Clemente-Napimoga, J.; Macedo, C.G.; Abdalla, H.B.; et al. Improved efficacy of naproxen-loaded NLC for temporomandibular joint administration. Sci. Rep. 2019, 9, 11160. [Google Scholar] [CrossRef] [Green Version]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafar, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, Q.; Zhang, J.; Huang, Y.; Deng, L.; Li, C.; Tai, G.; Ruan, B. Local penetration of doxorubicin via intrahepatic implantation of PLGA based doxorubicin-loaded implants. Drug Deliv. 2019, 26, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Madni, A.; Correia, A.; Rehman, M.; Balasubramanian, V.; Khan, M.M.; Santos, H.A. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int. J. Nanomed. 2019, 4961–4974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Jia, X.; Niu, H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018, 13, 4107–4119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenning, V.; Mäder, K.; Gohla, S.H. Solid lipid nanoparticles (SLN) based on binary mixtures of liquid and solid lipids: A (1)H-NMR study. Int. J. Pharm. 2000, 205, 15–21. [Google Scholar] [CrossRef]
- Castelli, F.; Puglia, C.; Sarpietro, M.G.; Rizza, L.; Bonina, F. Characterization of indomethacin-loaded lipid nanoparticles by differential scanning calorimetry. Int. J. Pharm. 2005, 304, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H. Surface-induced melting of small particles. Surf. Sci. 1996, 351, 285–291. [Google Scholar] [CrossRef]
- Antoniammal, P.; Arivuoli, D. Size and Shape Dependence on Melting Temperature of Gallium Nitride Nanoparticles. J. Nanomater. 2012, 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Hemn, H.O.; Saeed, M.I.; Yeap, S.K. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769–2781. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.V.; Seth, A.K.; Balaraman, R.; Aundhia, C.J.; Maheshwari, R.A.; Parmar, G.R. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo study. J. Adv. Res. 2016, 7, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [Green Version]
- Meirovitz, A.; Kuten, M.; Billan, S.; Abdah-Bortnyak, R.; Sharon, A.; Peretz, T.; Sela, M.; Schaffer, M.; Barak, V. Cytokines levels, Severity of acute mucositis and the need of PEG tube installation during chemo-radiation for head and neck cancer—A prospective pilot study. Radiat. Oncol. 2010, 16. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef]
- Thomsen, M.; Vitetta, L. Adjunctive Treatments for the Prevention of Chemotherapy- and Radiotherapy-Induced Mucositis. Integrative Cancer Therapies 2018, 17, 1027–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, C.; Mysara, M.; Claesen, J.; Baatout, S.; Leys, N.; Lebeer, S.; Verslegers, M.; Mastroleo, F. Intestinal mucositis precedes dysbiosis in a mouse model for pelvic irradiation. ISME Commun. 2021, 1, 24. [Google Scholar] [CrossRef]
- de Barros, P.A.V.; Andrade, M.E.R.; Generoso, S.d.V.; Miranda, S.E.M.; dos Reis, D.C.; Leocádio, P.C.L.; de Sales E Souza, E.L.; Martins, F.d.S.; da Gama, M.A.S.; Cassali, G.D.; et al. Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed. Pharmacother. 2018, 103, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.J.; Harmsen, H.J.M.; de Bont, E.S.J.M.; Tissing, W.J.E. The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis. PLoS Pathog. 2010, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; Scheerder, M.A.; Miinalainen, I.; Rappu, P.; Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2018, 69, 191–193. [Google Scholar] [CrossRef] [Green Version]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis, J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.K.; Lim, P.S.; Jin, J.S.; Wu, M.Y.; Chen, C.H. Impaired Gut Epithelial Tight Junction Expression in Hemodialysis Patients Complicated with Intradialytic Hypotension. BioMed Res. Int. 2018, 2018, 2670312. [Google Scholar] [CrossRef] [Green Version]
- Licona-Limón, P.; Henao-Mejia, J.; Temann, A.U.; Gagliani, N.; Licona-Limón, I.; Ishigame, H.; Hao, L.; Herbert, D.B.R.; Flavell, R.A. Th9 Cells Drive Host Immunity against Gastrointestinal Worm Infection. Immunity 2013, 39, 744–757. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Sonis, S.T. Pathobiology of mucositis. Semin. Oncol. Nurs. 2004, 20, 11–15. [Google Scholar] [CrossRef]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging Functions of Amphiregulin in Orchestrating Immunity, Inflammation, and Tissue Repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.E.; Morrison, P.J.; Wilhelm, C.; Wilson, M.; Ahlfors, H.; Renauld, J.C.; Panzer, U.; Helmby, H.; Stockinger, B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J. Exp. Med. 2013, 210, 2951–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Liu, Z.; Wu, W.; Rozo, C.; Bowdridge, S.; Millman, A.; Rooijen, N.V.; Urban, J.F., Jr.; Wynn, T.A.; Gause, W.C. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 2012, 18, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Osborne, L.C.; Noti, M.; Tran, S.V.; Zaiss, D.M.W.; Artis, D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin–EGFR interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 10762–10767. [Google Scholar] [CrossRef] [Green Version]







| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| ZO-1 | CCAGCTTATGAAAGGGTTGTTC | TCCTCTCTTGCCAACTTTTCTC |
| Occludin | ATGTCCGGCCGATGCTCTC | TTTGGCTGCTCTTGGGTCTGTAT |
| IL-33 | ATTTCCCCGGCAAAGTTCAG | AACGGAGTCTCATGCAGTAGA |
| TSLP | ACGGATGGGGCTAACTTACAA | AGTCCTCGATTTGCTCGAACT |
| IL-4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| IL-13 | CCTGGCTCTTGCTTGCCTT | GGTCTTGTGTGATGTTGCTCA |
| IL-22 | ATGAGTTTTTCCCTTATGGGGAC | GCTGGAAGTTGGACACCTCAA |
| IL-23 | ATGCTGGATTGCAGAGCAGTA | ACGGGGCACATTATTTTTAGTCT |
| IL-5 | CTCTGTTGACAAGCAATGAGACG | TCTTCAGTATGTCTAGCCCCTG |
| IL-25 | ACAGGGACTTGAATCGGGTC | TGGTAAAGTGGGACGACGGAGTTG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, C.M.; Horta, L.S.; Soares, A.P.; Carvalho, B.A.; Ferreira, E.; Lages, E.B.; Ferreira, L.A.M.; Faraco, A.A.G.; Santiago, H.C.; Goulart, G.A.C. Nanoencapsulated Doxorubicin Prevents Mucositis Development in Mice. Pharmaceutics 2021, 13, 1021. https://doi.org/10.3390/pharmaceutics13071021
Pinto CM, Horta LS, Soares AP, Carvalho BA, Ferreira E, Lages EB, Ferreira LAM, Faraco AAG, Santiago HC, Goulart GAC. Nanoencapsulated Doxorubicin Prevents Mucositis Development in Mice. Pharmaceutics. 2021; 13(7):1021. https://doi.org/10.3390/pharmaceutics13071021
Chicago/Turabian StylePinto, Cristiane M., Laila S. Horta, Amanda P. Soares, Bárbara A. Carvalho, Enio Ferreira, Eduardo B. Lages, Lucas A. M. Ferreira, André A. G. Faraco, Helton C. Santiago, and Gisele A. C. Goulart. 2021. "Nanoencapsulated Doxorubicin Prevents Mucositis Development in Mice" Pharmaceutics 13, no. 7: 1021. https://doi.org/10.3390/pharmaceutics13071021