Comparison of Modern In Vitro Permeability Methods with the Aim of Investigation Nasal Dosage Forms
Abstract
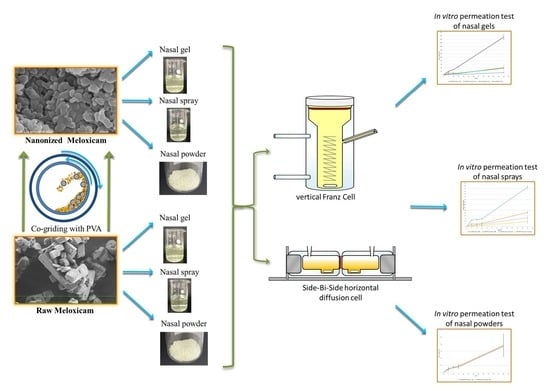
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Co-Ground Product
2.2.2. Characterization of Raw MX and Milled nanoMX
Size Distribution by Laser Diffraction
Scanning Electron Microscopy (SEM)
X-ray Powder Diffraction (XRPD)
2.2.3. Preparation of Intranasal Forms
2.2.4. Comparison of Hanson’s Vertical Diffusion Cell and Side-Bi-Side Horizontal Diffusion Cell
In Vitro Permeability on Hanson’s Vertical Diffusion Cell (Franz Cell) System
In Vitro Permeability on Side-Bi-Side Horizontal Diffusion Cell System
Determination of the Flux and the Permeability Coefficient
3. Results and Discussion
3.1. Characterization of Raw MX and Milled NanoMX
3.2. Comparison of the In Vitro Diffusion Methods
3.2.1. Investigation of Nasal Spray Forms
3.2.2. Investigation of Gel Forms
3.2.3. Investigation of Powder Forms
3.3. Evaluation of the Flux and the Permeability Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Saraf, S. Nose-to-brain drug delivery approach: A key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia Home Page. Available online: https://pheur.edqm.eu/home (accessed on 1 June 2021).
- Türker, S.; Onur, E.; Ózer, Y. Nasal route and drug delivery systems. Pharm. World Sci. 2004, 26, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kürti, L.; Kukovecz, Á.; Kozma, G.; Ambrus, R.; Deli, M.A.; Szabó-Révész, P. Study of the parameters influencing the co-grinding process for the production of meloxicam nanoparticles. Powder Technol. 2011, 212, 210–217. [Google Scholar] [CrossRef]
- Pomázi, A.; Buttini, F.; Ambrus, R.; Colombo, P.; Szabó-Révész, P. Effect of polymers for aerolization properties of mannitol-based microcomposites containing meloxicam. Eur. Polym. J. 2013, 49, 2518–2527. [Google Scholar] [CrossRef]
- Bartos, C.; Ambrus, R.; Sipos, P.; Budai-Szűcs, M.; Csányi, E.; Gáspár, R.; Márki, Á.; Seres, A.B.; Sztojkov-Ivanov, A.; Horváth, T.; et al. Study of sodium hyaluronate-based intranasal formulations containing micro- or nanosized meloxicam particles. Int. J. Pharm. 2015, 491, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Billotte, A.; Dunn, P.J.; Henry, B.T.; Marshall, P.V.; Woods, J.J. Intranasal Formulations for Treating Sexual Disorders. U.S. Patent Application No. 10/389,127, 21 August 2003. [Google Scholar]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Inoue, D.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.; Higaki, K.; Tanaka, A.; Yutani, R.; Sakane, T.; et al. Quantitative Estimation of the Effect of Nasal Mucociliary Function on in Vivo Absorption of Norfloxacin after Intranasal Administration to Rats. Mol. Pharm. 2018, 15, 4462–4469. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.K.; Keshari, R.K.; Rai, A.K. Advances in nasal trans-mucosal drug delivery. J. Appl. Pharm. Sci. 2011, 1, 21–28. [Google Scholar]
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef]
- Scherließ, R. Nasal formulations for drug administration and characterization of nasal preparations in drug delivery. Ther. Deliv. 2020, 11, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hasçiçek, C.; Gönül, N.; Erk, N. Mucoadhesive microspheres containing gentamicin sulfate for nasal administration: Preparation and in vitro characterization. Il Farm. 2003, 58, 11–16. [Google Scholar] [CrossRef]
- Anand, U.; Feridooni, T.; Agu, U.R. Novel Mucoadhesive Polymers for Nasal Drug Delivery. In Recent Advances in Novel Drug Carrier Systems; Sezer, A.D., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0810-8. [Google Scholar]
- Trenkel, M.; Scherließ, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef]
- Ambrus, R.; Gieszinger, P.; Gáspár, R.; Sztojkov-Ivanov, A.; Ducza, E.; Márki, Á.; Janáky, T.; Tömösi, F.; Kecskeméti, G.; Szabó-Révész, P.; et al. Investigation of the Absorption of Nanosized lamotrigine Containing Nasal Powder via the Nasal Cavity. Molecules 2020, 25, 1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartos, C.; Pallagi, E.; Szabó-Révész, P.; Ambrus, R.; Katona, G.; Kiss, T.; Rahimi, M.; Csóka, I. Formulation of levodopa containing dry powder for nasal delivery applying the quality-by-design approach. Eur. J. Pharm. Sci. 2018, 123, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Busato, L.; Pozzoli, M.; Ghadiri, M.; Ong, H.X.; Young, P.M.; Manfredini, S.; Traini, D. In vitro characterization of physico-chemical properties, cytotoxicity, bioactivity of urea-crosslinked hyaluronic acid and sodium ascorbyl phosphate nasal powder formulation. Int. J. Pharm. 2019, 558, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.C.; Peter, H.; Lang, S.R.; Ditzinger, G.; Merkle, H.P. In vitro cell models to study nasal mucosal permeability and metabolism. Adv. Drug Deliv. Rev. 1998, 29, 51–79. [Google Scholar] [CrossRef]
- Söderholm, J.D.; Hedman, L.; Artursson, P.; Franzen, L.; Larsson, J.; Pantzar, N.; Permert, J.; Olaison, G. Integrity and metabolism of human ileal mucosa in vitro in the Ussing chamber. Acta Physiol. Scand. 1998, 162, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.harvardapparatus.com/media/manuals/Product%20Manuals/6600xx_Navicyte_Manual.pdf (accessed on 1 June 2021).
- Watts, P.; Smith, A. PecSys: In situ gelling system for optimised nasal drug delivery. Expert Opin. Drug Deliv. 2009, 6, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Swamy, N.G.N.; Abbas, Z. Mucoadhesive in situ gels as nasal drug delivery systems: An overview. Asian J. Pharm. Sci. 2012, 7, 168–180. [Google Scholar]
- Bartos, C.; Ambrus, R.; Kovács, A.; Gáspár, R.; Sztojkov-Ivanov, A.; Márki, Á.; Janáky, T.; Tömösi, F.; Kecskeméti, G.; Szabó-Révész, P. Investigation of Absorption Routes of Meloxicam and Its Salt Form from Intranasal Delivery Systems. Molecules 2018, 23, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, T.; Ambrus, R.; Völgyi, G.; Budai-Szűcs, M.; Márki, Á.; Sipos, P.; Bartos, C.; Seres, A.B.; Sztojkov-Ivanov, A.; Takács-Novák, K.; et al. Effect of solubility enhancement on nasal absorption of meloxicam. Eur. J. Pharm. Sci. 2016, 95, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, T.; Ambrus, R.; Szabóné, R.P. Investigation of permeability of intranasal formulations using Side-Bi-Side horizontal diffusion cell. Acta Pharm. Hung. 2015, 85, 19–28. [Google Scholar]
- Hu, J.; Johnston, K.P.; Williams, R.O. Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev. Ind. Pharm. 2004, 30, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, D.E.; Zhang, G.G.Z.; Zhou, D.; Gao, Y.; Taylor, L.S. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm. Res. 2010, 27, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10, 95–108. [Google Scholar] [PubMed]
- Salamanca, C.; Barrera-Ocampo, A.; Lasso, J.; Camacho, N.; Yarce, C. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a static franz diffusion cell system for In Vitro permeation studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartos, C. Application of Wet Milling Techniques to Produced Micronized and Nanonized Drug Pre-Dispersions For The Development of Intranasal Formulations. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2016. [Google Scholar]
- Battistini, F.D.; Olivera, M.E.; Manzo, R.H. Equilibrium and release properties of hyaluronic acid–drug complexes. Eur. J. Pharm. Sci. 2013, 49, 588–594. [Google Scholar] [CrossRef]
- Borbás, E.; Balogh, A.; Bocz, K.; Müller, J.; Kiserdei, É.; Vigh, T.; Sinkó, B.; Marosi, A.; Halász, A.; Dohányos, Z.; et al. In vitro dissolution–permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μFluxTM. Int. J. Pharm. 2015, 491, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, W.M.; Price, J.C. Viscosity of polymer solution phase and other factors controlling the dissolution of theophylline microspheres prepared by the emulsion solvent evaporation method. J. Microencapsul. 2003, 20, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]






| Sample | NanoMX (mg/mL) | Raw MX (mg/mL) | PVP (mg/mL) | HA (mg/mL) |
|---|---|---|---|---|
| NanoMX powder | 1.0 | - | 1.0 | - |
| NanoMX spray | 1.0 | - | 1.0 | 1.0 |
| NanoMX gel | 1.0 | - | 1.0 | 5.0 |
| MX/PVP mix powder | - | 1.0 | 1.0 | - |
| MX/PVP mix spray | - | 1.0 | 1.0 | 1.0 |
| MX/PVP mix gel | - | 1.0 | 1.0 | 5.0 |
| Header | Franz Cell (Hanson Research Co., Los Angeles, CA, USA) | Side-Bi-Side Cell (Crown Glass, New York, NY, USA) |
|---|---|---|
| Relative position of the donor and acceptor chamber | vertical | horizontal |
| Surface of the diffusion | 1.8 cm2 | 0.69 cm2 |
| Volume of donor phase | 7 mL | 3 mL |
| Volume of acceptor phase | 0.3 mL | 3 mL |
| Place of sample administration | Directly to the membrane | Into the donor compartment |
| Franz Cell | Side-Bi-Side-Cell | |||
|---|---|---|---|---|
| Sample | J (µg/cm2/h) | Kp (cm/h) | J (µg/cm2/h) | Kp (cm/h) |
| NanoMX powder | - | - | 38.26 | 0.03826 |
| NanoMX spray | 16.20 | 0.01620 | 72.61 | 0.07261 |
| NanoMX gel | 212.44 | 0.21244 | 37.97 | 0.03797 |
| MX/PVP mix powder | - | - | 36.96 | 0.03696 |
| MX/PVP mix spray | 9.14 | 0.00914 | 25.93 | 0.02593 |
| MX/PVP mix gel | 40.90 | 0.04090 | 13.48 | 0.01348 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartos, C.; Szabó-Révész, P.; Horváth, T.; Varga, P.; Ambrus, R. Comparison of Modern In Vitro Permeability Methods with the Aim of Investigation Nasal Dosage Forms. Pharmaceutics 2021, 13, 846. https://doi.org/10.3390/pharmaceutics13060846
Bartos C, Szabó-Révész P, Horváth T, Varga P, Ambrus R. Comparison of Modern In Vitro Permeability Methods with the Aim of Investigation Nasal Dosage Forms. Pharmaceutics. 2021; 13(6):846. https://doi.org/10.3390/pharmaceutics13060846
Chicago/Turabian StyleBartos, Csilla, Piroska Szabó-Révész, Tamás Horváth, Patrícia Varga, and Rita Ambrus. 2021. "Comparison of Modern In Vitro Permeability Methods with the Aim of Investigation Nasal Dosage Forms" Pharmaceutics 13, no. 6: 846. https://doi.org/10.3390/pharmaceutics13060846