Pullulan Based Bioconjugates for Ocular Dexamethasone Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Pullulan-Dexamethasone
2.1.1. Synthesis of Carboxyethyl-Pullulan
- FT-IR (KBr). 3402 (-OH), 2930 (C-H), 1736 (O-C=O), 1638 (C-O-C) cm−1.
- Elemental analysis. Calcd: C, 45.02, H, 6.32%. Found: C, 40.96%; H, 6.15%.1H NMR (400 MHz, D2O). δ 5.45 (d, 1H, (1→4)-α-glycosidic bond), 5.00 (s, 1H, (1→6)-α-glycosidic bond), 4.56–3.27 [5H, remaining Hs of glucopyranose; 4H, 2x -CH2-, carboxyethyl group], 1.33 (t, 3H, -CH3, carboxyethyl group).
- 13C NMR (151 MHz, D2O). δ 172.32 (s, C=O, carboxyethyl group), 100.21, 99.74, 97.90, 80.11 (s, -CH2-, carboxyethyl group), 77.76, 77.14, 73.54, 73.41, 73.31, 73.04, 72.69, 71.69, 71.58, 71.48, 71.38, 71.31, 71.14, 70.68, 70.31, 70.06, 69.70, 69.44, 69.32, 68.43 (s, -CH2-, carboxyethyl group), 66.44, 62.33, 60.67, 60.37, 13.31 (s, -CH3, carboxyethyl group).
2.1.2. Synthesis of Carboxyhydrazide-Pullulan
- FT-IR (KBr). 3400 (-OH), 2929 (C-H), 1655 (N-C=O), 1638 (C-O-C) cm−1.
- Elemental analysis. Calcd: C, 43.55%, H, 6.15%, N, 2.42%. Found: C, 40.78%; H, 6.53%; N, 1.89%.
- 1H NMR (400 MHz, D2O). δ 5.42 (d, 1H, (1→4)-α-glycosidic bond), 5.00 (s, 1H, (1→6)-α-glycosidic bond), 4.53–3.36 [5H, remaining Hs of glucopyranose; 2H, -CH2-, carboxyethyl group].
- 13C NMR (101 MHz, D2O). δ 170.84 (s, C=O, carboxyethyl group), 100.25, 99.73, 97.96, 80.24 (s, -CH2-, carboxyethyl group), 77.87, 77.35, 73.81, 73.48, 73.32, 73.08, 72.52, 71.74, 71.63, 71.52, 71.43, 71.34, 71.20, 71.03, 70.36, 70.16, 69.79, 69.51, 69.38, 66.33, 62.37, 60.71, 60.44.
2.1.3. Synthesis of Pullulan-Dexamethasone
- FT-IR (KBr). 3408 (-OH), 2926 (C-H), 1654 (-C=O-NH-N=C), 1637 (C-O-C) cm−1.
- 1H NMR (400 MHz, D2O). δ 7.57 (d, 1Har, aromatic proton, dexamethasone), 6.47 (d, 1Har, dexamethasone), 6.27 (s, 1Har, dexamethasone), 5.41 (d, 1H, (1→4)-α-glycosidic bond), 4.99 (s, 1H, (1→6)-α-glycosidic bond), 4.60–3.40 (5H, remaining Hs of glucopyranose; 2H, -CH2-, carboxyethyl group; 12H, remaining Hs of dexamethasone), 1.59 (s, 3H, -CH3ar, dexamethasone), 1.02 (s, 3H, -C-CH3, dexamethasone), 0.92 (d, 3H, -CH-CH3, dexamethasone).
- 1H NMR (400 MHz, DMSO-d6, with internal standard 4-chloro-3-nitrobenzoic acid). δ 8.47 (d, 1Har, IS), 8.16 (dd, 1Har, IS), 7.95 (d, 1Har, dexamethasone), 7.88 (d, 1Har, IS), 5.65–2.99 [1H, (1→4)-α-glycosidic bond; 1H, (1→6)-α-glycosidic bond; 5H, remaining Hs of glucopyranose; 2H, -CH2-, carboxyethyl group; 12H, remaining Hs of dexamethasone], 1.24 (s, 3H, -CH3ar, dexamethasone), 1.17–1.00 (m, 3H, -C-CH3, dexamethasone), 0.97–0.74 (m, 3H, -CH-CH3, dexamethasone).
2.2. Synthesis of Pullulan-Dexamethasone-Cyanine3
- 1H NMR (400 MHz, DMSO-d6, with internal standard (4-chloro-3-nitrobenzoic acid). δ 8.33 (d, 1Har, IS), 8.07 (dd, 1Har, IS), 7.67 (d, 1Har, IS), 5.67–2.99 [1H, (1→4)-α-glycosidic bond; 1H, (1→6)-α-glycosidic bond; 5H, remaining Hs of glucopyranose; 2H, -CH2-, carboxyethyl group; 12H, remaining Hs of dexamethasone; 10H, 5x -CH2-, cyanine3], 2.67 (t, 3H, -CH3, cyanine3), 2.32 (q, 3H, -CH3, cyanine3), 1.24 (s, 3H, -CH3ar, dexamethasone), 1.18–1.03 (m, 3H, -C-CH3, dexamethasone), 0.96–0.73 (m, 3H, -CH-CH3, dexamethasone).
2.3. Synthesis of Pullulan-Cyanine3
- 1H NMR (400 MHz, D2O). δ 7.36 (s, 1Har, cyanine3), 5.43 (d, 1H, (1→4)-α-glycosidic bond), 5.00 (s, 1H, (1→6)-α-glycosidic bond), 4.60–3.40 [5H, remaining Hs of glucopyranose; 2H, -CH2-, carboxyethyl group; 10H, 5x -CH2-, cyanine3], 2.31 (s, 3H, -CH3, cyanine3), 2.09 (s, 3H, -CH3, cyanine3), 1.73 (s, 6H, 2x -CH3, cyanine3).
- 1H NMR (400 MHz, DMSO-d6, with internal standard (4-chloro-3-nitrobenzoic acid). δ 8.48 (d, 1Har, IS), 8.17 (dd, 1Har, IS), 7.90 (d, 1Har, IS), 6.63 (d, 1Har, cyanine3), 5.70–2.84 [1H, (1→4)-α-glycosidic bond; 1H, (1→6)-α-glycosidic bond; 5H, remaining Hs of glucopyranose; 2H, -CH2-, carboxyethyl group; 10H, 5x -CH2-, cyanine3], 2.67 (t, 3H, -CH3, cyanine3), 2.33 (q, 3H, -CH3, cyanine3).
2.4. Gel Permeation Chromatography
2.5. Size and Zeta Potential Analyses
2.6. Transmission Electron Microscopy (TEM)
2.7. Dexamethasone Release
2.8. Cytotoxicity Studies
2.9. Flow Cytometric Analysis
2.10. Confocal Microscopy
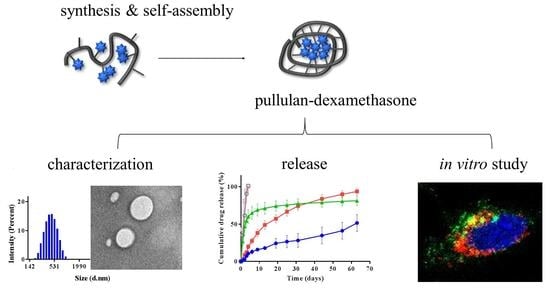
3. Results and Discussion
3.1. Synthesis of Bioconjugates
3.2. Colloidal Characterization
3.3. Dexamethasone Release
3.4. In Vitro Biocompatibility and Cell Association Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; De Smedt, S.C.; Remaut, K. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Veiga, F.; Silva, A.M.; Souto, E.B. Ocular drug delivery-new strategies for targeting anterior and posterior segments of the eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar] [CrossRef]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in ocular drug delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, L.; Sanders, N.N.; Braeckmans, K.; Boussery, K.; Van De Voorde, J.; De Smedt, S.C.; Demeester, J. Vitreous: A barrier to nonviral ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3553–3561. [Google Scholar] [CrossRef]
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.-Y.; Nance, E.A.; Yang, J.-C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J. Control. Release 2013, 167, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Kwon, I.C.; Kim, K.; et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials 2012, 33, 3485–3493. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kari, O.K.; Turunen, T.; Lajunen, T.; Schmitt, M.; Lehtinen, J.; Tasaka, F.; Parkkila, P.; Ndika, J.; Viitala, T.; et al. Diffusion and Protein Corona Formation of Lipid-Based Nanoparticles in the Vitreous Humor: Profiling and Pharmacokinetic Considerations. Mol. Pharm. 2020, 18, 699–713. [Google Scholar] [CrossRef]
- Mains, J.; Wilson, C.G. The vitreous humor as a barrier to nanoparticle distribution. J. Ocul. Pharmacol. Ther. 2013, 29, 143–150. [Google Scholar] [CrossRef]
- Mulchandani, A.; Luong, J.H.T.; Leduy, A. Biosynthesis of pullulan using immobilized Aureobasidium pullulans cells. Biotechnol. Bioeng. 1989, 33, 306–312. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Pullulan as a promising biomaterial for biomedical applications: A perspective. Trends Biomater. Artif. Organs 2007, 20, 116–121. [Google Scholar]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef]
- Na, K.; Bae, Y.H. Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: Characterization, aggregation, and adriamycin release in vitro. Pharm. Res. 2002, 19, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wen, X.; Liang, J.; Gu, Z.; Zhang, X.; Fan, Y. A pH-sensitive nano drug delivery system derived from pullulan/doxorubicin conjugate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 177–183. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Control. Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Bonzi, G.; Salmaso, S.; Scomparin, A.; Eldar-Boock, A.; Satchi-Fainaro, R.; Caliceti, P. Novel pullulan bioconjugate for selective breast cancer bone metastases treatment. Bioconjug. Chem. 2015, 26, 489–501. [Google Scholar] [CrossRef]
- Balasso, A.; Salmaso, S.; Pontisso, P.; Rosato, A.; Quarta, S.; Malfanti, A.; Mastrotto, F.; Caliceti, P. Re-programming pullulan for targeting and controlled release of doxorubicin to the hepatocellular carcinoma cells. Eur. J. Pharm. Sci. 2017, 103, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Balasso, A.; Subrizi, A.; Salmaso, S.; Mastrotto, F.; Garofalo, M.; Tang, M.; Chen, M.; Xu, H.; Urtti, A.; Caliceti, P. Screening of chemical linkers for development of pullulan bioconjugates for intravitreal ocular applications. Eur. J. Pharm. Sci. 2021, 161, 105785. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, G.; Zhang, J.; Lin, W.; Ji, F.; Bernards, M.T.; Chen, S. Development of zwitterionic polymer-based doxorubicin conjugates: Tuning the surface charge to prolong the circulation and reduce toxicity. Langmuir 2014, 30, 3764–3774. [Google Scholar] [CrossRef]
- Randárová, E.; Nakamura, H.; Islam, R.; Studenovský, M.; Mamoru, H.; Fang, J.; Chytil, P.; Etrych, T. Highly effective anti-tumor nanomedicines based on HPMA copolymer conjugates with pirarubicin prepared by controlled RAFT polymerization. Acta Biomater. 2020, 106, 256–266. [Google Scholar] [CrossRef]
- Howard, M.D.; Ponta, A.; Eckman, A.; Jay, M.; Bae, Y. Polymer micelles with hydrazone-ester dual linkers for tunable release of dexamethasone. Pharm. Res. 2011, 28, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Quan, L.-D.; Tian, J.; Laquer, F.C.; Ciborowski, P.; Wang, D. Syntheses of click PEG− dexamethasone conjugates for the treatment of rheumatoid arthritis. Biomacromolecules 2010, 11, 2621–2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, M.J.; Matson, J.B.; Tamboli, V.K.; Stupp, S.I. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials 2012, 33, 6823–6832. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Miller, S.C.; Liu, X.-M.; Anderson, B.; Wang, X.S.; Goldring, S.R. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res. Ther. 2007, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bílková, E.; Sedlák, M.; Imramovský, A.; Chárová, P.; Knotek, P.; Beneš, L. Prednisolone-α-cyclodextrin-star poly (ethylene glycol) polypseudorotaxane with delayed pH-sensitivity as a targeted drug delivery system. Int. J. Pharm. 2011, 414, 42–47. [Google Scholar] [CrossRef]
- Villanueva, J.R.; Villanueva, L.R.; Navarro, M.G. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int. J. Pharm. 2017, 516, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Bandello, F.; Loewenstein, A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin. Ophthalmol. 2015, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Pacella, F.; Ferraresi, A.F.; Turchetti, P.; Lenzi, T.; Giustolisi, R.; Bottone, A.; Fameli, V.; Romano, M.R.; Pacella, E. Intravitreal injection of Ozurdex® implant in patients with persistent diabetic macular edema, with six-month follow-up. Ophthalmol. Eye Dis. 2016, 8, 11–16. [Google Scholar] [CrossRef]
- Ratra, D.; Barh, A.; Banerjee, M.; Ratra, V.; Biswas, J. Safety and efficacy of intravitreal dexamethasone implant for refractory uveitic macular edema in adults and children. Ocul. Immunol. Inflamm. 2018, 26, 1034–1040. [Google Scholar] [CrossRef]
- Snyder, S.L.; Sobocinski, P.Z. An improved 2, 4, 6-trinitrobenzenesulfonic acid method for the determination of amines. Anal. Biochem. 1975, 64, 284–288. [Google Scholar] [CrossRef]
- Coman, A.G.; Paraschivescu, C.C.; Paun, A.; Diac, A.; Hădade, N.D.; Jouffret, L.; Gautier, A.; Matache, M.; Ionita, P. Synthesis of novel profluorescent nitroxides as dual luminescent-paramagnetic active probes. New J. Chem. 2017, 41, 7472–7480. [Google Scholar] [CrossRef]
- Vineberg, J.G.; Wang, T.; Zuniga, E.S.; Ojima, I. Design, synthesis, and biological evaluation of theranostic vitamin–linker–Taxoid conjugates. J. Med. Chem. 2015, 58, 2406–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.; Lu, H.; Tian, Y.; Li, H.; Wang, J.; Jin, Z. Structural modification and functional improvement of starch nanoparticles using vacuum cold plasma. Int. J. Biol. Macromol. 2020, 145, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Berman, E.R. Biochemistry of the Eye; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Bhattacharya, M.; Sadeghi, A.; Sarkhel, S.; Hagström, M.; Bahrpeyma, S.; Toropainen, E.; Auriola, S.; Urtti, A. Release of functional dexamethasone by intracellular enzymes: A modular peptide-based strategy for ocular drug delivery. J. Control. Rel. 2020, 327, 584–594. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicková, E.; Salmaso, S.; Mastrotto, F.; Caliceti, P.; Urtti, A. Pullulan Based Bioconjugates for Ocular Dexamethasone Delivery. Pharmaceutics 2021, 13, 791. https://doi.org/10.3390/pharmaceutics13060791
Kicková E, Salmaso S, Mastrotto F, Caliceti P, Urtti A. Pullulan Based Bioconjugates for Ocular Dexamethasone Delivery. Pharmaceutics. 2021; 13(6):791. https://doi.org/10.3390/pharmaceutics13060791
Chicago/Turabian StyleKicková, Eva, Stefano Salmaso, Francesca Mastrotto, Paolo Caliceti, and Arto Urtti. 2021. "Pullulan Based Bioconjugates for Ocular Dexamethasone Delivery" Pharmaceutics 13, no. 6: 791. https://doi.org/10.3390/pharmaceutics13060791