Property-Tuneable Microgels Fabricated by Using Flow-Focusing Microfluidic Geometry for Bioactive Agent Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GM
2.3. Structural Characterisation
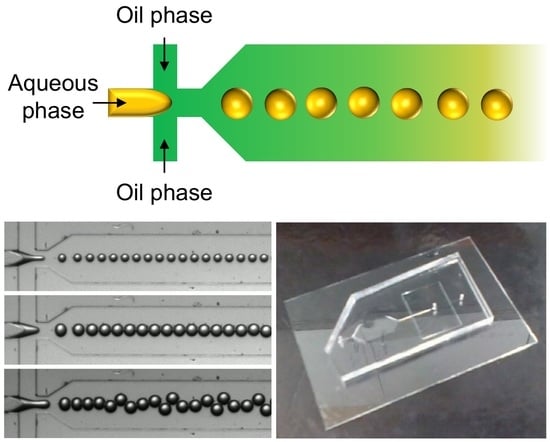
2.4. Generation of Microgels by Using a Microfluidic Flow-Focusing Device
2.5. Determination of the Swelling Behaviour
2.6. Thermogravimetric Analysis (TGA)
2.7. Evaluation of Cytotoxicity
2.8. Determination of the Encapsulation Efficiency
2.9. Evaluation of the Kinetics of Agent Release
2.10. Statistical Analysis
3. Results
3.1. Microgel Generation and Structural Characterisation
3.2. Thermal Properties and Size Control
3.3. Performance in Bioactive Agent Delivery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified gelatin nanoparticles for gene delivery. Int. J. Pharm. 2019, 554, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Hernaez-Moya, R.; Iturriaga, L.; Pedraz, J.L.; Lakshminarayanan, R.; Dolatshahi-Pirouz, A.; Taebnia, N.; Orive, G. Recent advances in gelatin-based therapeutics. Expert Opin. Biol. Ther. 2019, 19, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Occhetta, P.; Visone, R.; Russo, L.; Cipolla, L.; Moretti, M.; Rasponi, M. VA-086 methacrylate gelatine photopolymerizable hydrogels: A parametric study for highly biocompatible 3D cell embedding. J. Biomed. Mater. Res. A 2015, 103, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. Pt. A 2009, 15, 3221–3230. [Google Scholar] [CrossRef]
- Mehrali, M.; Thakur, A.; Kadumudi, F.B.; Pierchala, M.K.; Cordova, J.A.V.; Shahbazi, M.A.; Mehrali, M.; Pennisi, C.P.; Orive, G.; Gaharwar, A.K.; et al. Pectin methacrylate (PEMA) and gelatin-based hydrogels for cell delivery: Converting waste materials into biomaterials. ACS Appl. Mater. Int. 2019, 11, 12283–12297. [Google Scholar] [CrossRef]
- Puckert, C.; Tomaskovic-Crook, E.; Gambhir, S.; Wallace, G.G.; Crook, J.M.; Higgins, M.J. Molecular interactions and forces of adhesion between single human neural stem cells and gelatin methacrylate hydrogels of varying stiffness. Acta Biomater. 2020, 106, 156–169. [Google Scholar] [CrossRef]
- Ciortan, L.; Macarie, R.D.; Cecoltan, S.; Vadana, M.; Tucureanu, M.M.; Mihaila, A.C.; Droc, I.; Butoi, E.; Manduteanu, I. Chronic high glucose concentration induces inflammatory and remodeling changes in valvular endothelial cells and valvular interstitial cells in a gelatin methacrylate 3D model of the human aortic valve. Polymers 2020, 12, 2786. [Google Scholar] [CrossRef]
- Dupin, D.; Fujii, S.; Armes, S.P.; Reeve, P.; Baxter, S.M. Efficient synthesis of sterically stabilized pH-responsive microgels of controllable particle diameter by emulsion polymerization. Langmuir 2006, 22, 3381–3387. [Google Scholar] [CrossRef]
- Antonietti, M.; Bremser, W.; Mueschenborn, D.; Rosenauer, C.; Schupp, B.; Schmidt, M. Synthesis and size control of polystyrene latices via polymerization in microemulsion. Macromolecules 1991, 24, 6636–6643. [Google Scholar] [CrossRef]
- Antonietti, M.; Bremser, W.; Schmidt, M. Microgels: Model polymers for the crosslinked state. Macromolecules 1990, 23, 3796–3805. [Google Scholar] [CrossRef]
- Lai, W.F.; Susha, A.S.; Rogach, A.L.; Wang, G.A.; Huang, M.J.; Hu, W.J.; Wong, W.T. Electrospray-mediated preparation of compositionally homogeneous core-shell hydrogel microspheres for sustained drug release. RSC Adv. 2017, 7, 44482–44491. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.F.; Huang, E.; Wong, W.T. A gel-forming clusteroluminogenic polymer with tunable emission behavior as a sustained-release carrier enabling real-time tracking during bioactive agent delivery. Appl. Mater. Today 2020, 21, 100876. [Google Scholar] [CrossRef]
- Rajitha, K.; Mohana, K.N.S.; Mohanan, A.; Madhusudhana, A.M. Evaluation of anti-corrosion performance of modified gelatin-graphene oxide nanocomposite dispersed in epoxy coating on mild steel in saline media. Colloid Surf. A 2020, 587, 124341. [Google Scholar] [CrossRef]
- Lai, W.F.; Rogach, A.L.; Wong, W.T. One-pot synthesis of an emulsion-templated hydrogel-microsphere composite with tunable properties. Compos. Part A-Appl. Sci. Manuf. 2018, 113, 318–329. [Google Scholar] [CrossRef]
- Lai, W.F.; Tang, R.; Wong, W.T. Ionically crosslinked complex gels loaded with oleic acid-containing vesicles for transdermal drug delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Wong, E.; Wong, W.T. Multilayered composite-coated ionically crosslinked food-grade hydrogel beads generated from algal alginate for controlled and sustained release of bioactive compounds. RSC Adv. 2020, 10, 44522–44532. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T. Design of polymeric gene carriers for effective intracellular delivery. Trends Biotechnol. 2018, 36, 713–728. [Google Scholar] [CrossRef]
- Webber, M.J.; Pashuck, E.T. (Macro)molecular self-assembly for hydrogel drug delivery. Adv. Drug. Deliv. Rev. 2021, 172, 275–295. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.C.; Dalwadi, C.A. Recent patents on stimuli responsive hydrogel drug delivery system. Recent Pat. Drug. Deliv. Formul. 2013, 7, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug. Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Lai, W.F.; Shum, H.C. A stimuli-responsive nanoparticulate system using poly(ethylenimine)-graft-polysorbate for controlled protein release. Nanoscale 2016, 8, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a photosensitive material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buie, T.; McCune, J.; Cosgriff-Hernandez, E. Gelatin matrices for growth factor sequestration. Trends Biotechnol. 2020, 38, 546–557. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced graphene oxide incorporated GelMA hydrogel promotes angiogenesis for wound healing applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef] [Green Version]
- Dursun Usal, T.; Yucel, D.; Hasirci, V. A novel GelMA-pHEMA hydrogel nerve guide for the treatment of peripheral nerve damages. Int. J. Biol. Macromol. 2019, 121, 699–706. [Google Scholar] [CrossRef]







| Model | Microgel | |||
|---|---|---|---|---|
| MM80 | MM65 | MM50 | ||
| Zero-order model | r2 | 0.912 | 0.832 | 0.758 |
| First-order model | r2 | 0.985 | 0.980 | 0.984 |
| Higuchi model | r2 | 0.986 | 0.998 | 0.994 |
| Korsmeyer-Peppas model | r2 | 0.998 | 0.998 | 0.996 |
| n | 0.611 | 0.516 | 0.461 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.-F.; Wong, W.-T. Property-Tuneable Microgels Fabricated by Using Flow-Focusing Microfluidic Geometry for Bioactive Agent Delivery. Pharmaceutics 2021, 13, 787. https://doi.org/10.3390/pharmaceutics13060787
Lai W-F, Wong W-T. Property-Tuneable Microgels Fabricated by Using Flow-Focusing Microfluidic Geometry for Bioactive Agent Delivery. Pharmaceutics. 2021; 13(6):787. https://doi.org/10.3390/pharmaceutics13060787
Chicago/Turabian StyleLai, Wing-Fu, and Wing-Tak Wong. 2021. "Property-Tuneable Microgels Fabricated by Using Flow-Focusing Microfluidic Geometry for Bioactive Agent Delivery" Pharmaceutics 13, no. 6: 787. https://doi.org/10.3390/pharmaceutics13060787