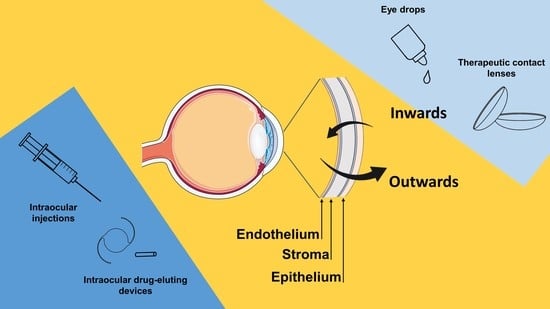
Asymmetry in Drug Permeability through the Cornea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Permeability Test
2.3. Drug Quantification
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matossian, C. Noncompliance with Prescribed Eyedrop Regimens Among Patients Undergoing Cataract Surgery—Prevalence, Consequences, and Solutions. US Ophthalmic Rev. 2020, 13, 18. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wong, T.T.; Mehta, J.S. Intraocular lens as a drug delivery reservoir. Curr. Opin. Ophthalmol. 2013, 24, 53–59. [Google Scholar] [CrossRef]
- Toffoletto, N.; Saramago, B.; Serro, A.P. Therapeutic Ophthalmic Lenses: A Review. Pharmaceutics 2021, 13, 36. [Google Scholar] [CrossRef]
- Lindstrom, R.; Kim, T. Ocular permeation and inhibition of retinal inflammation: An examination of data and expert opinion on the clinical utility of nepafenac. Curr. Med. Res. Opin. 2006, 22, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, N.; Bai, Z.; Xun, Y.; Jin, D.; Li, Z.; Cui, H. Effect of menthol on ocular drug delivery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1503–1510. [Google Scholar] [CrossRef]
- Ramsay, E.; del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, Y.; Wei, P.; Jhanji, V. Biomechanics and structure of the cornea: Implications and association with corneal disorders. Surv. Ophthalmol. 2018, 63, 851–861. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Serro, A.P.; Saramago, B. Dual drug delivery from intraocular lens material for prophylaxis of endophthalmitis in cataract surgery. Int. J. Pharm. 2019, 558, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.F.R.; Vieira, A.P.; Colaço, R.; Saramago, B.; Gil, M.H.; Coimbra, P.; Alves, P.; Bozukova, D.; Correia, T.R.; Correia, I.J.; et al. Controlled release of moxifloxacin from intraocular lenses modified by Ar plasma-assisted grafting with AMPS or SBMA: An in vitro study. Colloids Surf. B Biointerfaces 2017, 156, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Guerrero, V.; Zong, M.; Ramsay, E.; Rojas, B.; Sarkhel, S.; Gallego, B.; De Hoz, R.; Ramírez, A.I.; Salazar, J.J.; Triviño, A.; et al. Novel biodegradable polyesteramide microspheres for controlled drug delivery in Ophthalmology. J. Control. Release 2015, 211, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Haghjou, N.; Abdekhodaie, M.J.; Cheng, Y.L.; Saadatmand, M. Computer modeling of drug distribution after intravitreal administration. World Acad. Sci. Eng. Technol. 2011, 77, 706–716. [Google Scholar]
- Pimenta, A.F.R.; Serro, A.P.; Colaço, R.; Chauhan, A. Drug delivery to the eye anterior chamber by intraocular lenses: An in vivo concentration estimation model. Eur. J. Pharm. Biopharm. 2018, 133, 63–69. [Google Scholar] [CrossRef]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal Anti-inflammatory Drugs in Ophthalmology. Surv. Ophthalmol. 2010, 55, 108–133. [Google Scholar] [CrossRef]
- Gaynes, B. Topical ophthalmic NSAIDs: A discussion with focus on nepafenac ophthalmic suspension. Clin. Ophthalmol. 2008, 2, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, E.J.; Fingeret, M.; Mah, F.S. Use of Topical Steroids in Conjunctivitis: A Review of the Evidence. Cornea 2019, 38, 1062–1067. [Google Scholar] [CrossRef]
- Sarao, V.; Veritti, D.; Boscia, F.; Lanzetta, P. Intravitreal steroids for the treatment of retinal diseases. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Wielders, L.H.P.; Schouten, J.S.A.G.; Winkens, B.; van den Biggelaar, F.J.H.M.; Veldhuizen, C.A.; Murta, J.C.N.; Goslings, W.R.O.; Kohnen, T.; Tassignon, M.J.; Joosse, M.V.; et al. Randomized controlled European multicenter trial on the prevention of cystoid macular edema after cataract surgery in diabetics: ESCRS PREMED Study Report 2. J. Cataract Refract. Surg. 2018, 44, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef] [PubMed]
- OECD2020: Guidelines for the Testing of Chemicals, Section 4, Test N.437. Available online: https://www.oecd-ilibrary.org/environment/test-no-437-bovine-corneal-opacity-and-permeability-test-method-for-identifying-i-chemicals-inducing-serious-eye-damage-and-ii-chemicals-not-requiring-classification-for-eye-irritation-or-serious-eye-damage_978926 (accessed on 28 October 2020).
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef]
- Al-Ghabeish, M.; Xu, X.; Krishnaiah, Y.S.R.; Rahman, Z.; Yang, Y.; Khan, M.A. Influence of drug loading and type of ointment base on the in vitro performance of acyclovir ophthalmic ointment. Int. J. Pharm. 2015, 495, 783–791. [Google Scholar] [CrossRef]
- Prakash, K.; Sireesha, K.R. Liquid chromatographic method for determination of moxifloxacin and dexamethasone sodium phosphate in eye drops. Eurasian J. Anal. Chem. 2012, 7, 89–95. [Google Scholar]
- Pescina, S.; Govoni, P.; Potenza, A.; Padula, C.; Santi, P.; Nicoli, S. Development of a convenient ex vivo model for the study of the transcorneal permeation of drugs: Histological and permeability evaluation. J. Pharm. Sci. 2015, 104, 63–71. [Google Scholar] [CrossRef]
- Reer, O.; Bock, T.K.; Müller, B.W. in vitro corneal permeability of diclofenac sodium in formulations containing cyclodextrins compared to the commercial product voltaren ophtha. J. Pharm. Sci. 1994, 83, 1345–1349. [Google Scholar] [CrossRef]
- Gokce, E.H.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Güneri, T.; Caramella, C. Cyclosporine A loaded SLNs: Evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008, 364, 76–86. [Google Scholar] [CrossRef]
- Monti, D.; Saccomani, L.; Chetoni, P.; Burgalassi, S.; Saettone, M.F. Effect of iontophoresis on transcorneal permeation “in vitro” of two β-blocking agents, and on corneal hydration. Int. J. Pharm. 2003, 250, 423–429. [Google Scholar] [CrossRef]
- Dave, V.; Paliwal, S. A novel approach to formulation factor of aceclofenac eye drops efficiency evaluation based on physicochemical characteristics of in vitro and in vivo permeation. Saudi Pharm. J. 2014, 22, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic Acid in Soluplus® Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratieri, T.; Gelfuso, G.M.; Thomazini, J.A.; Lopez, R.F.V. Excised Porcine Cornea Integrity Evaluation in an in vitro Model of Iontophoretic Ocular Research. Ophthalmic Res. 2010, 43, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Malheiros, S.V.P.; De Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Interactions of ibuprofen with cationic polysaccharides in aqueous dispersions and hydrogels: Rheological and diffusional implications. Eur. J. Pharm. Sci. 2003, 20, 429–438. [Google Scholar] [CrossRef]
- Shah, A.; Khan, A.M.; Usman, M.; Siddiq, M. Thermodynamic Characterization of Dexamethasone Sodium Phosphate and its Complex With Dna As Studied By Conductometric and Spectroscopic Techniques. J. Chil. Chem. Soc. 2009, 2, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Kozlowska, M.; Rodziewicz, P.; Utesch, T.; Mroginski, M.A.; Kaczmarek-Kedziera, A. Solvation of diclofenac in water from atomistic molecular dynamics simulations-interplay between solute-solute and solute-solvent interactions. Phys. Chem. Chem. Phys. 2018, 20, 8629–8639. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Chauhan, A.; Srinivas, S.P. Penetration of fluorescein across the rabbit cornea from the endothelial surface. Pharm. Res. 2012, 29, 3325–3334. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin e loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Mishra, S.K.; Gupta, A.; Patyal, S.; Kumar, S.; Raji, K.; Singh, A.; Sharma, V. Intravitreal dexamethasone implant versus triamcinolone acetonide for macular oedema of central retinal vein occlusion: Quantifying efficacy and safety. Int. J. Retin. Vitr. 2018, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, J.A.; Bandello, F.; Belfort, R.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jiao, J.; Li, X.Y.; et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion: Twelve-month study results. Ophthalmology 2011, 118, 2453–2460. [Google Scholar] [CrossRef]
- Furino, C.; Boscia, F.; Niro, A.; Giancipoli, E.; Grassi, M.O.; D’Amico Ricci, G.; Blasetti, F.; Reibaldi, M.; Alessio, G. Combined Phacoemulsification and Intravitreal Dexamethasone Implant (Ozurdex®) in Diabetic Patients with Coexisting Cataract and Diabetic Macular Edema. J. Ophthalmol. 2017, 2017. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Ribeiro Filho, E.; Fialho, S.L.; Lucena, L.R.; Maia Filho, A.; Haddad, A.; Jorge, R.; Scott, I.U.; Da Silva Cunha, A. Pharmacokinetic and toxicity investigations of a new intraocular lens with a dexamethasone drug delivery system: A pilot study. Ophthalmologica 2006, 220, 338–342. [Google Scholar] [CrossRef]
- Dong, M.; Zhao, L.; Wang, F.; Hu, X.; Li, H.; Liu, T.; Zhou, Q.; Shi, W. Rapid porcine corneal decellularization through the use of sodium N-lauroyl glutamate and supernuclease. J. Tissue Eng. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, S.; Kamma-Lorger, C.S.; Boote, C.; Young, R.D.; Quantock, A.J.; Rost, A.; Khatib, Y.; Harris, J.; Yagi, N.; Terrill, N.; et al. The Effect of Riboflavin/UVA Collagen Cross-linking Therapy on the Structure and Hydrodynamic Behaviour of the Ungulate and Rabbit Corneal Stroma. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Yasueda, S.I.; Higashiyama, M.; Yamaguchi, M.; Isowaki, A.; Ohtori, A. Corneal critical barrier against the penetration of dexamethasone and lomefloxacin hydrochloride: Evaluation by the activation energy for drug partition and diffusion in cornea. Drug Dev. Ind. Pharm. 2007, 33, 805–811. [Google Scholar] [CrossRef]
- Zhang, W.; Prausnitz, M.R.; Edwards, A. Model of transient drug diffusion across cornea. J. Control. Release 2004, 99, 241–258. [Google Scholar] [CrossRef]
- Nagai, N.; Nakazawa, Y.; Ito, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. A nanoparticle-based ophthalmic formulation of dexamethasone enhances corneal permeability of the drug and prolongs its corneal residence time. Biol. Pharm. Bull. 2017, 40, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Awan, M.A.; Agarwal, P.K.; Watson, D.G.; McGhee, C.N.J.; Dutton, G.N. Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. Br. J. Ophthalmol. 2009, 93, 708–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buga, I.; Uzoma, J.I.; Reindel, K.; Rashid, K.; Diep, T.; McCartan, P.; Zhao, F. Physical and Chemical Stability of Dexamethasone Sodium Phosphate in Intravenous Admixtures Used to Prevent Chemotherapy-Induced Nausea and Vomiting. Hosp. Pharm. 2019. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.W.A. Drugs: Synonyms and Properties, 2nd ed.; Wiley, Ed.; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-0-566-08491-1. [Google Scholar]
- Malhotra, M.; Majumdar, D.K. Permeation through cornea. Indian J. Exp. Biol. 2001, 39, 11–24. [Google Scholar] [PubMed]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular delivery of macromolecules. J. Control. Release 2014, 190, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, J.; Chen, Z.J.; Sun, K.; Przekwas, A.; Walenga, R.; Fan, J. Computational modeling of drug transport across the in vitro cornea. Comput. Biol. Med. 2018, 92, 139–146. [Google Scholar] [CrossRef] [PubMed]





| Flux Direction | Drug Solution | Cumulative Mass Permeated after 6 h (µg/cm2) | tlag (h) | J (µg/(cm2 h)) | Pcoeff × 107 (cm/s) | R2 | |
|---|---|---|---|---|---|---|---|
| Bromfenac sodium | Inwards | Single | 1.3 ± 0.3 | 2.3 ± 0.2 | 0.34 ± 0.09 | 8 ± 2 | 0.94 ± 0.04 |
| Dual | 1.1 ± 0.2 | 2.3 ± 0.5 | 0.31 ± 0.05 | 7 ± 1 | 0.97 ± 0.02 | ||
| Outwards | Single | 2.61 ± 0.09 | 2.1 ± 0.2 | 0.55 ± 0.08 | 19 ± 3 | 0.94 ± 0.02 | |
| Dual | 2.1 ± 0.7 | 2.4 ± 0.4 | 0.59 ± 0.26 | 21 ± 9 | 0.97 ± 0.01 | ||
| Dexamethasone Sodium * | Inwards | Single | 0.25 ± 0.09 | 0 | 0.038 ± 0.006 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Dual | 0.16 ± 0.08 | 1 ± 1 | 0.04 ± 0.01 | 0.8 ± 0.3 | 0.8 ± 0.2 | ||
| Outwards | Single | 0.16 ± 0.01 | 0 | 0.02 ± 0.01 | 0.7 ± 0.3 | 0.8 ± 0.2 | |
| Dual | 0.12 ± 0.01 | 0.1 ± 4 | 0.03 ± 0.01 | 0.8 ± 0.4 | 0.87 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toffoletto, N.; Chauhan, A.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Asymmetry in Drug Permeability through the Cornea. Pharmaceutics 2021, 13, 694. https://doi.org/10.3390/pharmaceutics13050694
Toffoletto N, Chauhan A, Alvarez-Lorenzo C, Saramago B, Serro AP. Asymmetry in Drug Permeability through the Cornea. Pharmaceutics. 2021; 13(5):694. https://doi.org/10.3390/pharmaceutics13050694
Chicago/Turabian StyleToffoletto, Nadia, Anuj Chauhan, Carmen Alvarez-Lorenzo, Benilde Saramago, and Ana Paula Serro. 2021. "Asymmetry in Drug Permeability through the Cornea" Pharmaceutics 13, no. 5: 694. https://doi.org/10.3390/pharmaceutics13050694