Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Materials
2.2. Standard Solution Preparation
2.3. HPLC Instrumentation and Conditions
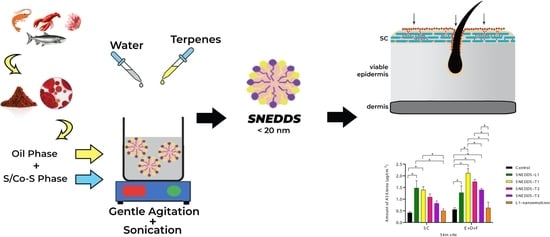
2.4. Design of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS)
2.5. Physical Characterization, Stability, and Antioxidant Activity
2.6. In Vitro Skin Penetration (IVPT) Study
Skin Distribution Study
2.7. Antioxidant Activity
2.8. Data Analysis
2.9. Statistical Analysis
3. Results
3.1. HPLC Assay Method Development
3.2. Preparation of ASX-Loaded SNEDDS
3.3. Physical Characteristics of ASX Nanoformulations
3.4. In Vitro Skin Penetration/Permeation Study
3.5. Effect of D-Limonene, Geraniol, and Farnesol Incorporated into SNEDDS on Skin Penetration
3.6. Preliminary Stability Study of ASX SNEDDS during Storage
3.7. Antioxidant Activity of ASX SNEDDS Formulations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos-e-Silva, M.; Celem, L.R.; Ramos-e-Silva, S.; Fucci-da-Costa, A.P. Anti-aging cosmetics: Facts and controversies. Clin. Dermatol. 2013, 31, 750–758. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Shah, M.; Mahfuzur, R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoso, A.; González, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 1–12. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases. Int. J. Mol. Med. 2020, 47, 37–48. [Google Scholar] [CrossRef]
- Xia, W.; Tang, N.; Varkaneh, H.K.; Low, T.Y.; Tan, S.C.; Wu, X.; Zhu, Y. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 1–11. [Google Scholar] [CrossRef]
- Fanaee-Danesh, E.; Gali, C.C.; Tadic, J.; Zandl-Lang, M.; Kober, A.C.; Agujetas, V.R.; de Dios, C.; Tam-Amersdorfer, C.; Stracke, A.; Albrecher, N.M. Astaxanthin exerts protective effects similar to bexarotene in Alzheimer’s disease by modulating amyloid-beta and cholesterol homeostasis in blood-brain barrier endothelial cells. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 2224–2245. [Google Scholar] [CrossRef] [PubMed]
- Cakir, E.; Cakir, U.; Tayman, C.; Turkmenoglu, T.T.; Gonel, A.; Turan, I.O. Favorable Effects of Astaxanthin on Brain Damage due to Ischemia-Reperfusion Injury. Comb. Chem. High Throughput Screen. 2020, 23, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Shohami, E.; Trembovler, V.; Heled, Y.; Horowitz, M. Cognitive effects of astaxanthin pretreatment on recovery from traumatic brain injury. Front. Neurol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Xu, L.; Yu, H.; Sun, H.; Yu, X.; Tao, Y. Optimized nonionic emulsifier for the efficient delivery of astaxanthin nanodispersions to retina: In vivo and ex vivo evaluations. Drug Deliv. 2019, 26, 1222–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2020, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, J.; Liu, J.; Sun, Z.; Wang, M.; Jiang, Y.; Chen, Z.-Y.; Chen, F. Antiaging Effects of Astaxanthin-Rich Alga Haematococcus pluvialis on Fruit Flies under Oxidative Stress. J. Agric. Food Chem. 2013, 61, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People-A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G. The xanthophyll carotenoid astaxanthin has distinct biological effects to prevent the photoaging of the skin even by its postirradiation treatment. Photochem. Photobiol. 2019, 95, 490–500. [Google Scholar] [CrossRef]
- Seki, T.; Sueki, H.; Kono, H.; Suganuma, K.; Yamashita, E. Effects of astaxanthin from Haematococcus pluvialis on human skin-patch test; skin repeated application test; effect on wrinkle reduction. Fragr. J. 2001, 12, 98–103. [Google Scholar]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with Dietary Astaxanthin Combined with Collagen Hydrolysate Improves Facial Elasticity and Decreases Matrix Metalloproteinase-1 and -12 Expression: A Comparative Study with Placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P.; Ling, T.C.; Lye, K.L.; Malmiri, H.J.; Nehdi, I.A.; Cheah, Y.K.; Mirhosseini, H.; Baharin, B.S. Effect of organic-phase solvents on physicochemical properties and cellular uptake of astaxanthin nanodispersions. J. Agric. Food Chem. 2011, 59, 8733–8741. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA J. Food 2018, 16, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Zhou, C.L.; Chen, F.P.; Han, D.; Wang, C.Y.; Li, J.X.; Chi, Z.; Liu, C.G. Development of a carboxymethyl chitosan functionalized nanoemulsion formulation for increasing aqueous solubility, stability and skin permeability of astaxanthin using low-energy method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Sun, R.; Xia, N.; Xia, Q. Non-aqueous nanoemulsions as a new strategy for topical application of astaxanthin. J. Dispers. Sci. Technol. 2019, 41, 1777–1788. [Google Scholar] [CrossRef]
- Eren, B.; Tuncay Tanriverdi, S.; Aydin Kose, F.; Ozer, O. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef]
- Geng, Q.; Zhao, Y.; Wang, L.; Xu, L.; Chen, X.; Han, J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. AAPS PharmSciTech 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Poorly Water-Soluble Talinolol: Preparation, in vitro and in vivo Assessment. Front. Pharmacol. 2019, 10, 459–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnaggar, Y.S.R.; Massik, M.A.E.; Abdallah, O.Y. Sildenafil citrate nanoemulsion vs. self-nanoemulsifying delivery systems: Rational development and transdermal permeation. Int. J. Nanotechnol 2011, 8, 749–763. [Google Scholar] [CrossRef]
- Badran, M.M.; Taha, E.I.; Tayel, M.M.; Al-Suwayeh, S.A. Ultra-fine self nanoemulsifying drug delivery system for transdermal delivery of meloxicam: Dependency on the type of surfactants. J. Mol. Liq. 2014, 190, 16–22. [Google Scholar] [CrossRef]
- van Staden, D.; du Plessis, J.; Viljoen, J. Development of Topical/Transdermal Self-Emulsifying Drug Delivery Systems, Not as Simple as Expected. Sci. Pharm. 2020, 88, 17. [Google Scholar] [CrossRef] [Green Version]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Khan, M.; Ali, M.; Shah, W.; Shah, A.; Yasinzai, M.M. Curcumin-loaded self-emulsifying drug delivery system (cu-SEDDS): A promising approach for the control of primary pathogen and secondary bacterial infections in cutaneous leishmaniasis. Appl. Microbiol. Biotechnol. 2019, 103, 7481–7490. [Google Scholar] [CrossRef]
- van Staden, D.; du Plessis, J.; Viljoen, J. Development of a Self-Emulsifying Drug Delivery System for Optimized Topical Delivery of Clofazimine. Pharmaceutics 2020, 12, 523. [Google Scholar] [CrossRef]
- El Maghraby, G.M. Self-microemulsifying and microemulsion systems for transdermal delivery of indomethacin: Effect of phase transition. Colloids Surf. B Biointerfaces 2010, 75, 595–600. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Chromatographic separation and purification of trans-astaxanthin from the extracts of Haematococcus pluvialis. J. Agric. Food Chem. 1998, 46, 3371–3375. [Google Scholar] [CrossRef]
- Cilurzo, F.; Minghetti, P.; Sinico, C. Newborn pig skin as model membrane in in vitro drug permeation studies: A technical note. AAPS PharmSciTech 2007, 8, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Nastiti, C.; Mohammed, Y.; Telaprolu, K.C.; Liang, X.; Grice, J.E.; Roberts, M.S.; Benson, H.A.E. Evaluation of Quantum Dot Skin Penetration in Porcine Skin: Effect of Age and Anatomical Site of Topical Application. Skin Pharmacol. Physiol. 2019, 32, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicology 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Davies, D.J.; Heylings, J.R.; McCarthy, T.J.; Correa, C.M. Development of an in vitro model for studying the penetration of chemicals through compromised skin. Toxicology 2015, 29, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In Vitro Antioxidant, Antityrosinase, and Cytotoxic Activities of Astaxanthin from Shrimp Waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.A.; Kazi, M.; Hadi Albgomi, M.; Ahad, A.; Raish, M. Development and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for curcumin transdermal delivery: An anti-inflammatory exposure. Drug Dev. Ind. Pharm. 2019, 45, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation (ICH). ICH Expert Working Group Harmonised Tripartite Guideline: Quality Risk Management Q8 (R2). Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf (accessed on 9 February 2021).
- International Council for Harmonisation (ICH). ICH Expert Working Group Harmonised Tripartite Guideline: Pharmaceutical Development Q9. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf (accessed on 9 February 2021).
- https://pharmaceutical.basf.com/. Kolliphor® EL. Available online: https://pharmaceutical.basf.com/global/en/drug-formulation/products/kolliphor-el.html (accessed on 9 February 2021).
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol(R)-Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.A.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Benson, H.A.; Sakran, W.; Roberts, M.S. Mechanistic evaluation of enhanced curcumin delivery through human skin in vitro from optimised nanoemulsion formulations fabricated with different penetration enhancers. Pharmaceutics 2019, 11, 639. [Google Scholar] [CrossRef] [Green Version]
- Fiume, M.Z. Final report on the safety assessment of triacetin. Int. J. Toxicol. 2003, 22 (Suppl. 2), 1–10. [Google Scholar]
- Kaur, R.; Ajitha, M. Transdermal delivery of fluvastatin loaded nanoemulsion gel: Preparation, characterization and in vivo anti-osteoporosis activity. Eur. J. Pharm. Sci. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Anton, N.; Gayet, P.; Benoit, J.-P.; Saulnier, P. Nano-emulsions and nanocapsules by the PIT method: An investigation on the role of the temperature cycling on the emulsion phase inversion. Int. J. Pharm. 2007, 344, 44–52. [Google Scholar] [CrossRef]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Terpenes and the Lipid–Protein–Partitioning Theory of Skin Penetration Enhancement. Pharm. Res. 1991, 8, 17–24. [Google Scholar] [CrossRef]
- National Library of Medicine: National Center for Biotechnology Information. PubChem Compound Summary for CID 440917, D-Limonene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/D-Limonene (accessed on 9 February 2021).
- National Library of Medicine: National Center for Biotechnology Information. PubChem Compound Summary for CID 445070, Geraniol. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/637566 (accessed on 9 February 2021).
- National Library of Medicine: National Center for Biotechnology Information. PubChem Compound Summary for CID 445070, Farnesol. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445070 (accessed on 9 February 2021).
- El-Kattan, A.F.; Asbill, C.S.; Michniak, B.B. The effect of terpene enhancer lipophilicity on the percutaneous permeation of hydrocortisone formulated in HPMC gel systems. Int. J. Pharm. 2000, 198, 179–189. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Novel Nanocarriers for Targeted Topical Skin Delivery of the Antioxidant Resveratrol. Pharmaceutics 2020, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- El-Kattan, A.F.; Asbill, C.S.; Kim, N.; Michniak, B.B. The effects of terpene enhancers on the percutaneous permeation of drugs with different lipophilicities. Int. J. Pharm. 2001, 215, 229–240. [Google Scholar] [CrossRef]





| Codes | ASX | Labrafil® M 1944 CS | Kolliphor® EL | Transcutol | D-Limonene | Geraniol | Farnesol | Water |
|---|---|---|---|---|---|---|---|---|
| SNEDDS–L1 | 0.003 | 1.00 | 4.00 | 1.00 | ||||
| SNEDDS–T1 | 0.003 | 0.95 | 4.00 | 1.00 | 0.05 | |||
| SNEDDS–T2 | 0.003 | 0.95 | 4.00 | 1.00 | 0.05 | |||
| SNEDDS–T3 | 0.003 | 0.95 | 4.00 | 1.00 | 0.05 | |||
| L1-NE | 0.006 | 1.00 | 4.00 | 1.00 | 6.00 |
| Codes | Droplet Size (nm) | PDI | Zeta Potential (mV) | Viscosity (Pas) | Refractive Index | pH | |
|---|---|---|---|---|---|---|---|
| Before Dilution | After Dilution | ||||||
| SNEDDS–L1 | 18.79 ± 0.54 | 0.24 ± 0.02 | −12.40 ± 0.20 | 0.19 ± 0.01 | 1.46 ± 0.01 | 8.01 ± 0.02 | 5.42 ± 0.01 |
| SNEDDS–T1 | 18.44 ± 0.41 | 0.21 ± 0.00 | −12.67 ± 0.21 | 0.19 ± 0.01 | 1.46 ± 0.01 | 8.01 ± 0.03 | 4.97 ± 0.04 |
| SNEDDS–T2 | 18.95 ± 1.48 | 0.25 ± 0.02 | −12.80 ± 0.35 | 0.20 ± 0.01 | 1.46 ± 0.01 | 7.97 ± 0.02 | 5.06 ± 0.03 |
| SNEDDS–T3 | 17.75 ± 0.21 | 0.26 ± 0.02 | −13.10 ± 0.52 | 0.23 ± 0.01 | 1.46 ± 0.01 | 7.89 ± 0.02 | 4.98 ± 0.08 |
| L1-NE | 87.13 ± 2.44 | 0.28 ± 0.00 | −12.53 ± 0.55 | 0.22 ± 0.01 | 1.40 ± 0.01 | 6.17 ± 0.01 | 4.43 ± 0.01 |
| Marketed ASX | 160.00 ± 3.44 | 0.20 ± 0.00 | −41.57 ± 2.46 | 0.01 ± 0.01 | 1.34 ± 0.01 | 5.40 ± 0.02 (no dilution) | |
| Codes | Droplet Size (nm) | ||
|---|---|---|---|
| 1:50 | 1:100 | 1:250 | |
| SNEDDS–L1 | 21.57 ± 0.05 | 18.79 ± 0.54 | 19.70 ± 0.27 |
| SNEDDS–T1 | 19.30 ± 1.33 | 18.44 ± 0.41 | 18.47 ± 0.32 |
| SNEDDS–T2 | 19.53 ± 1.35 | 18.95 ± 1.48 | 18.89 ± 0.50 |
| SNEDDS–T3 | 18.08 ± 0.78 | 17.75 ± 0.21 | 19.03 ± 0.99 |
| L1-NE | 88.00 ± 3.71 | 87.13 ± 2.44 | 84.44 ± 4.52 |
| Codes | ASX Distribution in the Skin (µg/cm2, mean ± SEM) | ER | |
|---|---|---|---|
| SC | E + D + F | ||
| Control: ASX in oil | 0.42 ± 0.01 | 0.56 ± 0.07 | 1.00 |
| Marketed ASX product | 0.67 ± 0.03 | 0.36 ± 0.03 | 1.05 |
| SNEDDS–L1 | 1.48 ± 0.31 | 1.28 ± 0.29 | 2.82 |
| SNEDDS–T1 | 1.40 ± 0.14 | 2.12 ± 0.19 | 3.59 |
| SNEDDS–T2 | 1.09 ± 0.14 | 1.75 ± 0.10 | 2.90 |
| SNEDDS–T3 | 0.82 ± 0.10 | 1.40 ± 0.06 | 2.27 |
| L1–NE | 0.50 ± 0.08 | 0.63 ± 0.24 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponto, T.; Latter, G.; Luna, G.; Leite-Silva, V.R.; Wright, A.; Benson, H.A.E. Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery. Pharmaceutics 2021, 13, 649. https://doi.org/10.3390/pharmaceutics13050649
Ponto T, Latter G, Luna G, Leite-Silva VR, Wright A, Benson HAE. Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery. Pharmaceutics. 2021; 13(5):649. https://doi.org/10.3390/pharmaceutics13050649
Chicago/Turabian StylePonto, Thellie, Gemma Latter, Giuseppe Luna, Vânia R. Leite-Silva, Anthony Wright, and Heather A. E. Benson. 2021. "Novel Self-Nano-Emulsifying Drug Delivery Systems Containing Astaxanthin for Topical Skin Delivery" Pharmaceutics 13, no. 5: 649. https://doi.org/10.3390/pharmaceutics13050649