Supercritical CO2 Extraction of Organic Solvents from Flunisolide and Fluticasone Propionate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Flunisolide
3.1.1. Effect of Pressure
3.1.2. Effect of Temperature
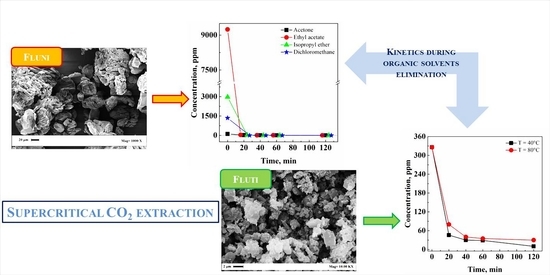
3.1.3. Extraction Kinetics
3.2. Fluticasone Propionate
3.2.1. Effect of Pressure
3.2.2. Effect of Temperature and Extraction Kinetics
3.3. Other Characterizations
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- XXX USP. <467> General Chapter. In Organic Volatile Impurities; Pharmacopeial Forum: Rockville, MD, USA, 2007. [Google Scholar]
- B’Hymer, C. Residual solvent testing: A review of gas-chromatographic and alternative techniques. Pharm. Res. 2003, 20, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12. [Google Scholar] [PubMed]
- Vandezande, P.; Gevers, L.E.M.; Vankelecom, I.F.J. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef] [PubMed]
- Geens, J.; De Witte, B.; Van der Bruggen, B. Removal of API’s (active pharmaceutical ingredients) from organic solvents by nanofiltration. Sep. Sci. Technol. 2007, 42, 2435–2449. [Google Scholar] [CrossRef]
- White, L.S. Development of large-scale applications in organic solvent nanofiltration and pervaporation for chemical and refining processes. J. Membr. Sci. 2006, 286, 26–35. [Google Scholar] [CrossRef]
- De Marco, I.; Rossmann, M.; Prosapio, V.; Reverchon, E.; Braeuer, A. Control of particle size, at micrometric and nanometric range, using supercritical antisolvent precipitation from solvent mixtures: Application to PVP. Chem. Eng. J. 2015, 273, 344–352. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Supercritical assisted electrospray: An improved micronization process. Polymers 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obaidat, R.; Aleih, H.; Mashaqbeh, H.; Altaani, B.; Alsmadi, M.M.; Alnaief, M. Development and evaluation of cocoa butter taste masked ibuprofen using supercritical carbon dioxide. AAPS Pharm. Sci. Tech. 2021, 22, 106. [Google Scholar] [CrossRef]
- Cardea, S.; Pisanti, P.; Reverchon, E. Generation of chitosan nanoporous structures for tissue engineering applications using a supercritical fluid assisted process. J. Supercrit. Fluids 2010, 54, 290–295. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. SC-CO2-assisted process for a high energy density aerogel supercapacitor: The effect of GO loading. Nanotechnology 2017, 28, 204001. [Google Scholar] [CrossRef]
- Sarver, J.A.; Kiran, E. Foaming of polymers with carbon dioxide—The year-in-review—2019. J. Supercrit. Fluids 2021, 173, 105166. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Carius, B.; Simões, M.M.Q.; Portugal, I.; Saraiva, J.; Silva, C.M. Supercritical CO2 extraction of V. vinifera leaves: Influence of cosolvents and particle size on removal kinetics and selectivity to target compounds. J. Supercrit. Fluids 2020, 165, 104959. [Google Scholar] [CrossRef]
- Ishak, I.; Hussain, N.; Coorey, R.; Ghani, M.A. Optimization and characterization of chia seed (Salvia hispanica L.) oil extraction using supercritical carbon dioxide. J. CO2 Utiliz. 2021, 45, 101430. [Google Scholar] [CrossRef]
- Liu, W.-W.; Li, M.-Z.; Short, T.; Qing, X.-C.; He, Y.-M.; Li, Y.-Z.; Liu, L.-H.; Zhang, H.; Zhang, H.-C. Supercritical carbon dioxide cleaning of metal parts for remanufacturing industry. J. Clean. Prod. 2015, 93, 339–346. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Biodegradable membranes loaded with curcumin to be used as engineered independent devices in active packaging. J. Taiwan Inst. Chem. Eng. 2017, 71, 518–526. [Google Scholar] [CrossRef]
- Su, W.; Zhang, H.; Xing, Y.; Li, X.; Wang, J.; Cai, C. A bibliometric analysis and review of supercritical fluids for the synthesis of nanomaterials. Nanomaterials 2021, 11, 336. [Google Scholar] [CrossRef]
- Hariyanto, P.; Myint, A.A.; Kim, J. Complete drying and micronization of ecamsule using supercritical CO2 as the antisolvent. J. Supercrit. Fluids 2021, 170, 105157. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. Supercritical CO2 processing to improve the electrochemical properties of graphene oxide. J. Supercrit. Fluids 2016, 118, 119–127. [Google Scholar] [CrossRef]
- Baldino, L.; Della Porta, G.; Reverchon, E. Supercritical CO2 processing strategies for pyrethrins selective extraction. J. CO2 Utiliz. 2017, 20, 14–19. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Chang, C.J.; Day, C.Y.; Ko, C.M.; Chiu, K.L. Densities and P-x-y diagrams for carbon dioxide dissolution in methanol, ethanol, acetone mixtures. Fluid Phase Equilib. 1997, 131, 243–258. [Google Scholar] [CrossRef]
- Byun, H.S.; Choi, M.Y.; Lim, J.S. High pressure phase behaviour and modelling of binary mixtures for alkyl acetate in supercritical carbon dioxide. J. Supercrit. Fluids 2006, 37, 323–332. [Google Scholar] [CrossRef]
- Vega Gonzalez, A.; Tufeu, R.; Subra, P. High-pressure vapor-liquid equilibrium for the binary systems carbon dioxide-dimethyl sulfoxide and carbon dioxide-dichloromethane. J. Chem. Eng. Data 2002, 47, 492–495. [Google Scholar] [CrossRef]
- Reighard, T.S.; Lee, S.T.; Olesik, S.V. Determination of methanol/CO2 and acetonitrile/CO2 vapor-liquid phase equilibria using a variable-volume view cell. Fluid Phase Equilib. 1996, 123, 215–230. [Google Scholar] [CrossRef]
- Pöhler, H.; Kiran, E. Volumetric properties of carbon dioxide + acetone at high pressures. J. Chem. Eng. Data 1997, 42, 379–383. [Google Scholar] [CrossRef]
- Pöhler, H.; Kiran, E. Volumetric properties of carbon dioxide + ethanol at high pressures. J. Chem. Eng. Data 1997, 42, 384–388. [Google Scholar] [CrossRef]
- Yeo, S.-D.; Park, S.-J.; Kim, J.-W.; Kim, J.-C. Critical properties of carbon dioxide + methanol, + ethanol, + 1-propanol, and + 1-butanol. J. Chem. Eng. Data 2000, 45, 932–935. [Google Scholar] [CrossRef]
- Fábián, B.; Horvai, G.; Idrissi, A.; Jedlovszky, P. Vapour-liquid equilibrium of acetone-CO2 mixtures of different compositions at the vicinity of the critical point. J. CO2 Utiliz. 2019, 34, 465–471. [Google Scholar] [CrossRef]
- Velaga, S.P.; Berger, R.; Carlfors, J. Supercritical fluids crystallization of budesonide and flunisolide. Pharm. Res. 2002, 19, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Vatanara, A.; Rouholamini, N.A.; Gilani, K.; Asgharian, R.; Darabi, M.; Rafiee, T.M. Precipitation of fluticasone propionate microparticles using supercritical antisolvent. DARU J. Pharm. Sci. 2009, 17, 6–12. [Google Scholar]
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide, 1st ed.; CRC Press: London, UK, 2006. [Google Scholar]
- Ammar, H.O.; Ghorab, M.M.; Mahmoud, A.A.; Shahin, H.I. Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. AAPS Pharm. Sci. Tech. 2017, 18, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| API | Acetone, ppm (Class 3) | Ethyl Acetate 1, ppm (Class 3) | Isopropyl Ether, ppm (Class 3) | Dichloromethane 1, ppm (Class 2) | Initial Overall Organic Solvent Residues, ppm |
|---|---|---|---|---|---|
| Fluni | 110 | 9244 | 2970 | 1347 | 13,671 |
| Fluti | 326 | - | - | - | 326 |
| API | Acetone, ppm (Class 3) | Ethyl Acetate, ppm (Class 3) | Isopropyl Ether, ppm (Class 3) | Dichloromethane, ppm (Class 2) | Final Overall Organic Solvent Residues, ppm |
|---|---|---|---|---|---|
| Fluni | 4 | 8 | 0 | 0 | 12 |
| Fluti | 10 | - | - | - | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical CO2 Extraction of Organic Solvents from Flunisolide and Fluticasone Propionate. Pharmaceutics 2021, 13, 612. https://doi.org/10.3390/pharmaceutics13050612
Baldino L, Scognamiglio M, Reverchon E. Supercritical CO2 Extraction of Organic Solvents from Flunisolide and Fluticasone Propionate. Pharmaceutics. 2021; 13(5):612. https://doi.org/10.3390/pharmaceutics13050612
Chicago/Turabian StyleBaldino, Lucia, Mariarosa Scognamiglio, and Ernesto Reverchon. 2021. "Supercritical CO2 Extraction of Organic Solvents from Flunisolide and Fluticasone Propionate" Pharmaceutics 13, no. 5: 612. https://doi.org/10.3390/pharmaceutics13050612