Polymeric Caffeic Acid Acts as a Nasal Vaccine Formulation against Streptococcus pneumoniae Infections in Mice
Abstract
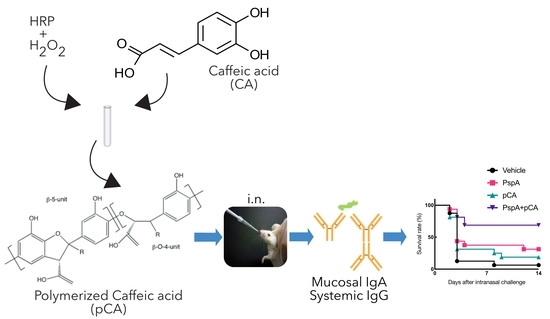
:1. Introduction
2. Materials and Methods
2.1. Animals and Materials
2.2. Preparation of pCA
2.3. Preparation of PspA Expression Plasmid
2.4. Purification of Recombinant PspA Protein
2.5. Immunization and Sampling Schedule for the Assessment of PspA-Specific Antibody Production
2.6. ELISA for Detection of PspA-Specific Antibody
2.7. In vivo Pneumococcal Infection Study
2.8. Statistical Analysis
3. Results
3.1. Nasal Immunization of a PspA Antigen with pCA Elicits PspA-Specific Antibody Responses in the Mucosal and Systemic Compartments
3.2. Nasal Vaccination with PspA and pCA Confers Protective Immunity against a Lethal Dose of Pneumococcal Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fauci, A.S.; Touchette, N.A.; Folkers, G.K. Emerging Infectious Diseases: A 10-Year Perspective from the National Institute of Allergy and Infectious Diseases. Emerg. Infect. Dis. 2005, 11, 519–525. [Google Scholar] [CrossRef]
- Yang, L.; Grenfell, B.T.; Mina, M.J. Waning Immunity and Re-Emergence of Measles and Mumps in the Vaccine Era. Curr. Opin. Virol. 2020, 40, 48–54. [Google Scholar] [CrossRef]
- Henriques-Normark, B.; Tuomanen, E.I. The Pneumococcus: Epidemiology, Microbiology, and Pathogenesis. Csh. Perspect. Med. 2013, 3, a010215. [Google Scholar] [CrossRef]
- Hoshi, S.; Kondo, M.; Okubo, I. Economic Evaluation of Immunisation Programme of 23-Valent Pneumococcal Polysaccharide Vaccine and the Inclusion of 13-Valent Pneumococcal Conjugate Vaccine in the List for Single-Dose Subsidy to the Elderly in Japan. PLoS ONE 2015, 10, e0139140. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of Disease Caused by Streptococcus Pneumoniae in Children Younger than 5 Years: Global Estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Calix, J.J.; Nahm, M.H. A New Pneumococcal Serotype, 11E, Has a Variably Inactivated WcjE Gene. J. Infect. Dis. 2010, 202, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Calix, J.J.; Porambo, R.J.; Brady, A.M.; Larson, T.R.; Yother, J.; Abeygunwardana, C.; Nahm, M.H. Biochemical, Genetic, and Serological Characterization of Two Capsule Subtypes among Streptococcus Pneumoniae Serotype 20 Strains: DISCOVERY OF A NEW PNEUMOCOCCAL SEROTYPE. J. Biol. Chem. 2012, 287, 27885–27894. [Google Scholar] [CrossRef] [Green Version]
- Langereis, J.D.; Jonge, M.I. de Non-Encapsulated Streptococcus Pneumoniae, Vaccination as a Measure to Interfere with Horizontal Gene Transfer. Virulence 2017, 8, 637–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiberman, A.; Dagan, R.; Leibovitz, E.; Yagupsky, P.; Fliss, D.M. The Bacteriology of the Nasopharynx in Childhood. Int. J. Pediatr. Otorhinolaryngol. 1999, 49, S151–S153. [Google Scholar] [CrossRef]
- Obukhanych, T.V.; Nussenzweig, M.C. T-Independent Type II Immune Responses Generate Memory B Cells. J. Exp. Med. 2006, 203, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondada, S.; Wu, H.-J.; Robertson, D.A.; Chelvarajan, R.L. Accessory Cell Defect in Unresponsiveness of Neonates and Aged to Polysaccharide Vaccines. Vaccine 2000, 19, 557–565. [Google Scholar] [CrossRef]
- Hollingshead, S.K.; Becker, R.; Briles, D.E. Diversity of PspA: Mosaic Genes and Evidence for Past Recombination in Streptococcus Pneumoniae. Infect. Immun. 2000, 68, 5889–5900. [Google Scholar] [CrossRef] [Green Version]
- Yun, K.W.; Choi, E.H.; Lee, H.J. Genetic Diversity of Pneumococcal Surface Protein A in Invasive Pneumococcal Isolates from Korean Children, 1991–2016. PLoS ONE 2017, 12, e0183968. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.; Kim, S.Y.; Kim, M.S.; Lee, S.E.; Rhee, J.H. Intranasal Immunization with Recombinant PspA Fused with a Flagellin Enhances Cross-Protective Immunity against Streptococcus Pneumoniae Infection in Mice. Vaccine 2011, 29, 5731–5739. [Google Scholar] [CrossRef]
- Nabors, G.S.; Braun, P.A.; Herrmann, D.J.; Heise, M.L.; Pyle, D.J.; Gravenstein, S.; Schilling, M.; Ferguson, L.M.; Hollingshead, S.K.; Briles, D.E.; et al. Immunization of Healthy Adults with a Single Recombinant Pneumococcal Surface Protein A (PspA) Variant Stimulates Broadly Cross-Reactive Antibodies to Heterologous PspA Molecules. Vaccine 2000, 18, 1743–1754. [Google Scholar] [CrossRef]
- Kong, I.G.; Sato, A.; Yuki, Y.; Nochi, T.; Takahashi, H.; Sawada, S.; Mejima, M.; Kurokawa, S.; Okada, K.; Sato, S.; et al. Nanogel-Based PspA Intranasal Vaccine Prevents Invasive Disease and Nasal Colonization by Streptococcus Pneumoniae. Infect. Immun. 2013, 81, 1625–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KUNISAWA, J.; GOHDA, M.; KIYONO, H. Uniqueness of the Mucosal Immune System for the Development of Prospective Mucosal Vaccine. Yakuga Zasshi 2007, 127, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Kondoh, M.; Yagi, K.; Kiyono, H.; Kunisawa, J. The Development of Mucosal Vaccine Using Bacterial Function for Targeting Mucosal Tissues. Yakuga Zasshi 2014, 134, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ada, G. Vaccines and Vaccination. N. Engl. J. Med. 2001, 345, 1042–1053. [Google Scholar] [CrossRef]
- Borges, O.; Lebre, F.; Bento, D.; Borchard, G.; Junginger, H.E. Mucosal Vaccines: Recent Progress in Understanding the Natural Barriers. Pharmaceut. Res. 2010, 27, 211–223. [Google Scholar] [CrossRef]
- Yamanaka, D.; Tajima, K.; Adachi, Y.; Ishibashi, K.-I.; Miura, N.N.; Motoi, M.; Ohno, N. Effect of Polymeric Caffeic Acid on Antitumour Activity and Natural Killer Cell Activity in Mice. J. Funct. Food 2014, 6, 513–522. [Google Scholar] [CrossRef]
- Yamanaka, D.; Motoi, M.; Ishibashi, K.-I.; Miura, N.N.; Adachi, Y.; Ohno, N. Modulation of Interferon-γ Synthesis by the Effects of Lignin-like Enzymatically Polymerized Polyphenols on Antigen-Presenting Cell Activation and the Subsequent Cell-to-Cell Interactions. Food Chem. 2013, 141, 4073–4080. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, D.; Ishibashi, K.-I.; Adachi, Y.; Ohno, N. Species Difference in Reactivity to Lignin-like Enzymatically Polymerized Polyphenols on Interferon-γ Synthesis and Involvement of Interleukin-2 Production in Mice. Int. Immunopharmacol. 2016, 38, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, D.; Tamiya, Y.; Motoi, M.; Ishibashi, K.-I.; Miura, N.N.; Adachi, Y.; Ohno, N. The Effect of Enzymatically Polymerised Polyphenols on CD4 Binding and Cytokine Production in Murine Splenocytes. PLoS ONE 2012, 7, e36025. [Google Scholar] [CrossRef] [Green Version]
- Tada, R.; Yamanaka, D.; Ogasawara, M.; Saito, M.; Ohno, N.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Polymeric Caffeic Acid Is a Safer Mucosal Adjuvant That Augments Antigen-Specific Mucosal and Systemic Immune Responses in Mice. Mol. Pharmaceut. 2018, 15, 4226–4234. [Google Scholar] [CrossRef]
- Tada, R.; Ogasawara, M.; Yamanaka, D.; Sakurai, Y.; Negishi, Y.; Kiyono, H.; Ohno, N.; Kunisawa, J.; Aramaki, Y. Enzymatically Polymerised Polyphenols Prepared from Various Precursors Potentiate Antigen-Specific Immune Responses in Both Mucosal and Systemic Compartments in Mice. PLoS ONE 2021, 16, e0246422. [Google Scholar] [CrossRef]
- Tada, R.; Suzuki, H.; Takahashi, S.; Negishi, Y.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Nasal Vaccination with Pneumococcal Surface Protein A in Combination with Cationic Liposomes Consisting of DOTAP and DC-Chol Confers Antigen-Mediated Protective Immunity against Streptococcus Pneumoniae Infections in Mice. Int. Immunopharmacol. 2018, 61, 385–393. [Google Scholar] [CrossRef]
- Suzuki, H.; Watari, A.; Hashimoto, E.; Yonemitsu, M.; Kiyono, H.; Yagi, K.; Kondoh, M.; Kunisawa, J. C-Terminal Clostridium Perfringens Enterotoxin-Mediated Antigen Delivery for Nasal Pneumococcal Vaccine. PLoS ONE 2015, 10, e0126352. [Google Scholar] [CrossRef] [Green Version]
- Okegawa, Y.; Motohashi, K. A Simple and Ultra-Low Cost Homemade Seamless Ligation Cloning Extract (SLiCE) as an Alternative to a Commercially Available Seamless DNA Cloning Kit. Biochem. Biophys. Rep. 2015, 4, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Okegawa, Y.; Motohashi, K. Evaluation of Seamless Ligation Cloning Extract Preparation Methods from an Escherichia Coli Laboratory Strain. Anal. Biochem. 2015, 486, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Muto, S.; Iwata, T.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Attachment of Class B CpG ODN onto DOTAP/DC-Chol Liposome in Nasal Vaccine Formulations Augments Antigen-Specific Immune Responses in Mice. BMC Res. Notes 2017, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, R.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Intranasal Administration of Cationic Liposomes Enhanced Granulocyte–Macrophage Colony-Stimulating Factor Expression and This Expression Is Dispensable for Mucosal Adjuvant Activity. BMC Res. Notes 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Tada, R.; Ohshima, A.; Tanazawa, Y.; Ohmi, A.; Takahashi, S.; Kiyono, H.; Kunisawa, J.; Aramaki, Y.; Negishi, Y. Essential Role of Host Double-Stranded DNA Released from Dying Cells by Cationic Liposomes for Mucosal Adjuvanticity. Vaccines 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Gonzalez, R.; Guo, L.; Wu, C.; Wu, J.; Vernet, G.; Paranhos-Baccalà, G.; Wang, J.; Hung, T. Large-Scale Seroprevalence Analysis of Human Metapneumovirus and Human Respiratory Syncytial Virus Infections in Beijing, China. Virol. J. 2011, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Gillgrass, A.E.; Ashkar, A.A.; Rosenthal, K.L.; Kaushic, C. Prolonged Exposure to Progesterone Prevents Induction of Protective Mucosal Responses Following Intravaginal Immunization with Attenuated Herpes Simplex Virus Type 2. J. Virol. 2003, 77, 9845–9851. [Google Scholar] [CrossRef] [Green Version]
- Briles, D.E.; Ades, E.; Paton, J.C.; Sampson, J.S.; Carlone, G.M.; Huebner, R.C.; Virolainen, A.; Swiatlo, E.; Hollingshead, S.K. Intranasal Immunization of Mice with a Mixture of the Pneumococcal Proteins PsaA and PspA Is Highly Protective against Nasopharyngeal Carriage of Streptococcus Pneumoniae. Infect. Immun. 2000, 68, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Finkelman, F.D.; Holmes, J.; Katona, I.M.; Jr, J.F.U.; Beckmann, M.P.; Park, L.S.; Schooley, K.A.; Coffman, R.L.; Mosmann, T.R.; Paul, W.E. Lymphokine Control of in Vivo Immunoglobulin Isotype Selection. Annu. Rev. Immunol. 1990, 8, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; King, J.D.; Kataoka, K.; Kobayashi, R.; Gilbert, R.S.; Oishi, K.; Hollingshead, S.K.; Briles, D.E.; Fujihashi, K. Secretory-IgA Antibodies Play an Important Role in the Immunity to Streptococcus Pneumoniae. J. Immunol. 2010, 185, 1755–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.; Vishukumar, A. Host Soluble Mediators: Defying the Immunological Inertness of Aspergillus Fumigatus Conidia. J. Fungi 2018, 4, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, A.-H.T.; Fulgham, R.L.; McCrory, M.A.; Briles, D.E.; Szalai, A.J. Pneumococcal Surface Protein A Inhibits Complement Activation by Streptococcus Pneumoniae. Infect. Immun. 1999, 67, 4720–4724. [Google Scholar] [CrossRef] [Green Version]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Bourvellec, C.L.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, R.; Suzuki, H.; Ogasawara, M.; Yamanaka, D.; Adachi, Y.; Kunisawa, J.; Negishi, Y. Polymeric Caffeic Acid Acts as a Nasal Vaccine Formulation against Streptococcus pneumoniae Infections in Mice. Pharmaceutics 2021, 13, 585. https://doi.org/10.3390/pharmaceutics13040585
Tada R, Suzuki H, Ogasawara M, Yamanaka D, Adachi Y, Kunisawa J, Negishi Y. Polymeric Caffeic Acid Acts as a Nasal Vaccine Formulation against Streptococcus pneumoniae Infections in Mice. Pharmaceutics. 2021; 13(4):585. https://doi.org/10.3390/pharmaceutics13040585
Chicago/Turabian StyleTada, Rui, Hidehiko Suzuki, Miki Ogasawara, Daisuke Yamanaka, Yoshiyuki Adachi, Jun Kunisawa, and Yoichi Negishi. 2021. "Polymeric Caffeic Acid Acts as a Nasal Vaccine Formulation against Streptococcus pneumoniae Infections in Mice" Pharmaceutics 13, no. 4: 585. https://doi.org/10.3390/pharmaceutics13040585