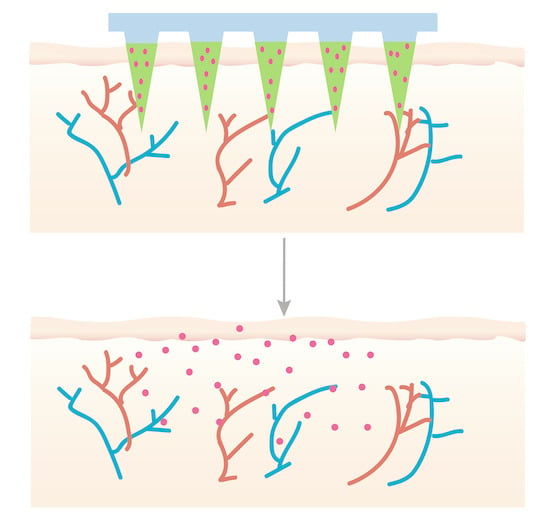
Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blank and Drug-Loaded Dissolving MNs
2.3. Morphology of MNs
2.4. Mechanical Property of MNs
2.5. In Vitro Skin Insertion Tests
2.6. In Vivo Dissolution of MNs after Insertion
2.7. In Vivo Skin Recovery after MNs Insertion
2.8. Drug Release from MNs
2.9. In Vivo IVIS Image
2.10. Ex Vivo Skin Retention and Drug Permeation
2.11. HPLC Methods and Drug Loading
2.12. Data Analysis
3. Results and Discussion
3.1. Morphology of the MNs and Drug Distribution
3.2. The Mechanical Strength of the MNs
3.3. In Vitro Skin Insertion Tests
3.4. In Vivo Dissolution of the MNs after Insertion and the Recovery of the Skin
3.5. In Vivo Skin Recovery after MNs Insertion
3.6. Loading of Propranolol Hydrochloride in the MNs
3.7. Drug Release from MNs
3.8. In Vivo IVIS Image
3.9. Permeation and Skin Retention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aqil, M.; Sultana, Y.; Ali, A. Transdermal delivery of beta-blockers. Expert Opin. Drug Deliv. 2006, 3, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Alany, R. Topical and transdermal formulation and drug delivery. Pharm. Dev. Technol. 2017, 22, 457. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Leone, M.; Mönkäre, J.; Bouwstra, J.A.; Kersten, G. Dissolving microneedle patches for dermal vaccination. Pharm. Res. 2017, 34, 2223–2240. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Li, W.; Zhou, X.; Yang, F.; Qian, Z. Microneedles-based transdermal drug delivery systems: A review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597. [Google Scholar] [CrossRef]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001, 46, 281–305. [Google Scholar] [CrossRef]
- Zuo, J.; Du, L.; Li, M.; Liu, B.; Zhu, W.; Jin, Y. Transdermal enhancement effect and mechanism of iontophoresis for non-steroidal anti-inflammatory drugs. Int. J. Pharm. 2014, 466, 76–82. [Google Scholar] [CrossRef]
- Kanikkannan, N. Iontophoresis-based transdermal delivery systems. BioDrugs 2002, 16, 339–347. [Google Scholar] [CrossRef]
- Oberli, M.A.; Schoellhammer, C.M.; Langer, R.; Blankschtein, D. Ultrasound-enhanced transdermal delivery: Recent advances and future challenges. Ther. Deliv. 2014, 5, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Seah, B.C.; Teo, B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Park, H.; Seo, J.; Lee, S. Sonophoresis in transdermal drug deliverys. Ultrasonics 2014, 54, 56–65. [Google Scholar] [CrossRef]
- Murthy, S.N.; Sammeta, S.M.; Bowers, C. Magnetophoresis for enhancing transdermal drug delivery: Mechanistic studies and patch design. J. Control. Release 2010, 148, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Sammeta, S.M.; Repka, M.A.; Murthy, S.N. Magnetophoresis in combination with chemical enhancers for transdermal drug delivery. Drug Dev. Ind. Pharm. 2011, 37, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Zhang, Y.; Gu, Z. Punching and electroporation for enhanced transdermal drug delivery. Theranostics 2018, 8, 3688–3690. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Huang, Y.; Li, Z. Transdermal delivery of nucleic acid mediated by punching and electroporation. Methods Mol. Biol. 2020, 2050, 101–112. [Google Scholar]
- Chen, X.; Zhu, L.; Li, R.; Pang, L.; Zhu, S.; Ma, J.; Du, L.; Jin, Y. Electroporation-enhanced transdermal drug delivery: Effects of logp, pk(a), solubility and penetration time. Eur. J. Pharm. Sci. 2020, 151, 105410. [Google Scholar] [CrossRef]
- Wong, T.W. Electrical, magnetic, photomechanical and cavitational waves to overcome skin barrier for transdermal drug delivery. J. Control. Release 2014, 193, 257–269. [Google Scholar] [CrossRef]
- Lee, S.; McAuliffe, D.J.; Kollias, N.; Flotte, T.J.; Doukas, A.G. Permeabilization and recovery of the stratum corneum in vivo: The synergy of photomechanical waves and sodium lauryl sulfate. Lasers Surg. Med. 2001, 29, 145–150. [Google Scholar] [CrossRef]
- Lee, S.; Kollias, N.; McAuliffe, D.J.; Flotte, T.J.; Doukas, A.G. Topical drug delivery in humans with a single photomechanical wave. Pharm. Res. 1999, 16, 1717–1721. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Y.; Qi, J.; Zhu, Q.; Lu, Y.; Wu, W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today 2018, 23, 181–186. [Google Scholar] [CrossRef]
- Pireddu, R.; Schlich, M.; Marceddu, S.; Valenti, D.; Pini, E.; Fadda, A.M.; Lai, F.; Sinico, C. Nanosuspensions and microneedles roller as a combined approach to enhance diclofenac topical bioavailability. Pharmaceutics 2020, 12, 1140. [Google Scholar] [CrossRef]
- Ramaut, L.; Hoeksema, H.; Pirayesh, A.; Stillaert, F.; Monstrey, S. Microneedling: Where do we stand now? A systematic review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Milewski, M.; Dick, L.; Zhang, J.; Bothe, J.R.; Gehrt, M.; Manser, K.; Nissley, B.; Petrescu, I.; Johnson, P.; et al. Coated microneedles for transdermal delivery of a potent pharmaceutical peptide. Biomed. Microdevices 2019, 22, 7. [Google Scholar] [CrossRef]
- Chen, Z.; He, J.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y. Long-acting microneedles: A progress report of the state-of-the-art techniques. Drug Discov. Today 2020, 25, 1462–1468. [Google Scholar] [CrossRef]
- Ita, K. Dissolving microneedles for transdermal drug delivery: Advances and challenges. Biomed. Pharmacother. 2017, 93, 1116–1127. [Google Scholar] [CrossRef]
- Li, J.; Zeng, M.; Shan, H.; Tong, C. Microneedle patches as drug and vaccine delivery platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Maurya, A.; Nanjappa, S.H.; Honnavar, S.; Salwa, M.; Murthy, S.N. Rapidly dissolving microneedle patches for transdermal iron replenishment therapy. J. Pharm. Sci. 2018, 107, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Yang, F.; Liu, J.; Hu, H.; Du, X.; Hanagata, N.; Zhao, S.; Zhu, Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater. Sci. 2018, 6, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; McCaffrey, J.; Ali, A.A.; McBride, J.W.; McCrudden, C.M.; Vincente-Perez, E.M.; Donnelly, R.F.; McCarthy, H.O. Dissolving microneedles for DNA vaccination: Improving functionality via polymer characterization and rala complexation. Hum. Vaccin. Immunother. 2017, 13, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Liu, H.M.; Zhou, J.; Wang, Y.G.; Feng, X.; Tang, H.; Yan, Q.; Zhu, R.S.; Wu, Y.W.; Wang, X.G.; et al. Skin test of tuberculin purified protein derivatives with a dissolving microneedle-array patch. Drug Deliv. Transl. Res. 2019, 9, 795–801. [Google Scholar] [CrossRef]
- Yang, H.; Kang, G.; Jang, M.; Um, D.J.; Shin, J.; Kim, H.; Hong, J.; Jung, H.; Ahn, H.; Gong, S.; et al. Development of lidocaine-loaded dissolving microneedle for rapid and efficient local anesthesia. Pharmaceutics 2020, 12, 1067. [Google Scholar] [CrossRef]
- Fu, D.G. Cardiac arrhythmias: Diagnosis, symptoms, and treatments. Cell Biochem. Biophys. 2015, 73, 291–296. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Dumas de la Roque, E.; Hubiche, T.; Boralevi, F.; Thambo, J.B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Leaute-Labreze, C.; Harper, J.I.; Hoeger, P.H. Infantile haemangioma. Lancet 2017, 390, 85–94. [Google Scholar] [CrossRef]
- Wedgeworth, E.; Glover, M.; Irvine, A.D.; Neri, I.; Baselga, E.; Clayton, T.H.; Beattie, P.E.; Bjerre, J.V.; Burrows, N.P.; Foelster-Holst, R.; et al. Propranolol in the treatment of infantile haemangiomas: Lessons from the european propranolol in the treatment of complicated haemangiomas (pitch) taskforce survey. Br. J. Dermatol. 2016, 174, 594–601. [Google Scholar] [CrossRef]
- Al-Haddad, C.; El Salloukh, N.A.; El Moussawi, Z. Β-blockers in the treatment of periocular infantile hemangioma. Curr. Opin. Ophthalmol. 2019, 30, 319–325. [Google Scholar] [CrossRef]
- Marey, H.M.; Elmazar, H.F.; Mandour, S.S.; Khairy, H.A. Combined oral and topical beta blockers for the treatment of early proliferative superficial periocular infantile capillary hemangioma. J. Pediatr. Ophthalmol. Strabismus. 2018, 55, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Zaher, H.; Rasheed, H.; Esmat, S.; Hegazy, R.A.; Gawdat, H.I.; Hegazy, R.A.; El-Komy, M.; Abdelhalim, D.M. Propranolol and infantile hemangiomas: Different routes of administration, a randomized clinical trial. Eur. J. Dermatol. 2013, 23, 646–652. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Bakheit, A.H.H.; Abdel Aziz, H.A.; Alajmi, F.M.; AlRabiah, H. Propranolol. Profiles Drug Subst. Excip. Relat. Methodol. 2017, 42, 287–338. [Google Scholar]
- Kovačević, M.; Lukinović Škudar, V.; Maričić, G.; Krnjević-Pezić, G.; Stanimirović, A. Topical propranolol cream in treatment of superficial infantile hemangiomas: A literature review and 4 years of clinical experience. Acta Derm. Alp. Pannonica Adriat. 2014, 23, 75–78. [Google Scholar] [CrossRef]
- Price, A.; Rai, S.; McLeod, R.W.J.; Birchall, J.C.; Elhassan, H.A. Topical propranolol for infantile haemangiomas: A systematic review. J. Eur. Acad. Dermatol Venereol. 2018, 32, 2083–2089. [Google Scholar] [CrossRef]
- Da Silva Marques, Z.T.; Santos-Oliveira, R.; de Oliveira de Siqueira, L.B.; Cardoso, V.D.S.; de Freitas, Z.M.F.; Barros, R.; Villa, A.L.V.; Monteiro, M.; Dos Santos, E.P.; Ricci-Junior, E. Development and characterization of a nanoemulsion containing propranolol for topical delivery. Int. J. Nanomed. 2018, 13, 2827–2837. [Google Scholar] [CrossRef] [Green Version]
- Pamornpathomkul, B.; Ngawhirunpat, T.; Tekko, I.A.; Vora, L.; McCarthy, H.O.; Donnelly, R.F. Dissolving polymeric microneedle arrays for enhanced site-specific acyclovir delivery. Eur. J. Pharm. Sci. 2018, 121, 200–209. [Google Scholar] [CrossRef]
- An, M.; Liu, H. Dissolving microneedle arrays for transdermal delivery of amphiphilic vaccines. Small 2017, 13, 1700164. [Google Scholar] [CrossRef]
- Dillon, C.; Hughes, H.; O’Reilly, N.J.; Allender, C.J.; Barrow, D.A.; McLoughlin, P. Dissolving microneedle based transdermal delivery of therapeutic peptide analogues. Int. J. Pharm. 2019, 565, 9–19. [Google Scholar] [CrossRef]
- Kang, G.; Tu, T.N.T.; Kim, S.; Yang, H.; Jang, M.; Jo, D.; Ryu, J.; Baek, J.; Jung, H. Adenosine-loaded dissolving microneedle patches to improve skin wrinkles, dermal density, elasticity and hydration. Int. J. Cosmet. Sci. 2018, 40, 199–206. [Google Scholar] [CrossRef]
- Bok, M.; Zhao, Z.J.; Hwang, S.H.; Kang, H.J.; Jeon, S.; Ko, J.; Jeong, J.; Song, Y.S.; Lim, E.; Jeong, J.H. Effective dispensing methods for loading drugs only to the tip of DNA microneedles. Pharmaceutics 2020, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Tarbox, T.N.; Watts, A.B.; Cui, Z.; Williams, R.O., 3rd. An update on coating/manufacturing techniques of microneedles. Drug Deliv. Transl. Res. 2018, 8, 1828–1843. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Monteiro-Riviere, N.A.; Lusk, J.; Morel, P.; Minghetti, P.; Lenardi, C.; Chichkov, B.N.; Narayan, R.J. Fabrication of polymer microneedles using a two-photon polymerization and micromolding process. J. Diabetes Sci. Technol. 2009, 3, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, M.S.; Zafar, S.; Zahra, A.T.; Zaman, M.H.; Akhtar, A.; Kucuk, I.; Farhan, M.; Chang, M.W.; Ahmad, Z. Fabrication and characterisation of self-applicating heparin sodium microneedle patches. J. Drug Target. 2021, 29, 60–68. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Bankar, N.G.; Kulkarni, M.V.; Venuganti, V.V.K. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4t1 xenografted breast cancer mouse model. Int. J. Pharm. 2019, 556, 263–275. [Google Scholar] [CrossRef]
- Sadeqi, A.; Nejad, H.R.; Kiaee, G.; Sonkusale, S. Cost-effective fabrication of chitosan microneedles for transdermal drug delivery. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 5737–5740. [Google Scholar]
- Chen, Y.W.; Chen, M.C.; Wu, K.W.; Tu, T.Y. A facile approach for rapid prototyping of microneedle molds, microwells and micro-through-holes in various substrate materials using co (2) laser drilling. Biomedicines 2020, 8, 427. [Google Scholar] [CrossRef]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases: A mini review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Du, H.; Liu, P.; Zhu, J.; Lan, J.; Li, Y.; Zhang, L.; Zhu, J.; Tao, J. Hyaluronic acid-based dissolving microneedle patch loaded with methotrexate for improved treatment of psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kwon, H.J.; Ahn, G.R.; Ko, E.J.; Yoo, K.H.; Kim, B.J.; Lee, C.; Kim, D. Hyaluronic acid microneedle patch for the improvement of crow’s feet wrinkles. Dermatol. Ther. 2017, 30, e12546. [Google Scholar] [CrossRef]
- Swathi, H.P.; Anusha Matadh, V.; Paul Guin, J.; Narasimha Murthy, S.; Kanni, P.; Varshney, L.; Suresh, S.; Shivakumar, H.N. Effect of gamma sterilization on the properties of microneedle array transdermal patch system. Drug Dev. Ind. Pharm. 2020, 46, 606–620. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. Hpmc/pvp dissolving microneedles: A promising delivery platform to promote trans-epidermal delivery of alpha-arbutin for skin lightening. AAPS PharmSciTech 2019, 21, 25. [Google Scholar] [CrossRef]
- Balmert, S.C.; Carey, C.D.; Falo, G.D.; Sethi, S.K.; Erdos, G.; Korkmaz, E.; Falo, L.D., Jr. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J. Control. Release 2020, 317, 336–346. [Google Scholar] [CrossRef]
- Lee, I.C.; Lin, W.M.; Shu, J.C.; Tsai, S.W.; Chen, C.H.; Tsai, M.T. Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice. J Biomed. Mater. Res. A 2017, 105, 84–93. [Google Scholar] [CrossRef]
- Yao, W.; Tao, C.; Zou, J.; Zheng, H.; Zhu, J.; Zhu, Z.; Zhu, J.; Liu, L.; Li, F.; Song, X. Flexible two-layer dissolving and safing microneedle transdermal of neurotoxin: A biocomfortable attempt to treat rheumatoid arthritis. Int. J. Pharm. 2019, 563, 91–100. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, M.I.; Siddique, M.I.; Sarwar, H.S.; Shahnaz, G.; Hussain, S.Z.; Bukhari, N.I.; Hussain, I.; Sohail, M.F. Fabrication and characterization of thiolated chitosan microneedle patch for transdermal delivery of tacrolimus. AAPS PharmSciTech 2020, 21, 68. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-pd1 antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The effects of geometry on skin penetration and failure of polymer microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef] [Green Version]
- Ita, K. Reflections on the insertion and fracture forces of microneedles. Curr. Drug Deliv. 2017, 14, 357–363. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Lee, S.; Lahiji, S.F.; Jang, J.; Jang, M.; Jung, H. Micro-pillar integrated dissolving microneedles for enhanced transdermal drug delivery. Pharmaceutics 2019, 11, 402. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.M.; Lee, C.; Lahiji, S.F.; Jung, U.W.; Chung, G.; Jung, H. Dissolving microneedles for rapid and painless local anesthesia. Pharmaceutics 2020, 12, 366. [Google Scholar] [CrossRef] [Green Version]
- Dong, P.; Teutloff, C.; Lademann, J.; Patzelt, A.; Schäfer-Korting, M.; Meinke, M.C. Solvent effects on skin penetration and spatial distribution of the hydrophilic nitroxide spin probe pca investigated by epr. Cell Biochem. Biophys. 2020, 78, 127–137. [Google Scholar] [CrossRef]
- Das, C.; Olmsted, P.D. The physics of stratum corneum lipid membranes. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2016, 374, 20150126. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride. Pharmaceutics 2021, 13, 579. https://doi.org/10.3390/pharmaceutics13040579
He J, Zhang Z, Zheng X, Li L, Qi J, Wu W, Lu Y. Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride. Pharmaceutics. 2021; 13(4):579. https://doi.org/10.3390/pharmaceutics13040579
Chicago/Turabian StyleHe, Jingjing, Zichen Zhang, Xianzi Zheng, Lu Li, Jianping Qi, Wei Wu, and Yi Lu. 2021. "Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride" Pharmaceutics 13, no. 4: 579. https://doi.org/10.3390/pharmaceutics13040579