Development, Characterization, and Ex Vivo Assessment of Elastic Liposomes for Enhancing the Buccal Delivery of Insulin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
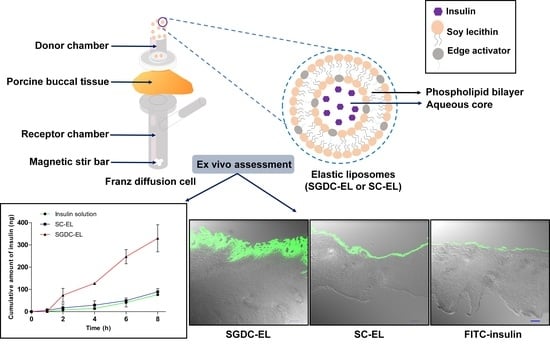
2.2. Preparation of Elastic Liposomes
2.3. Particle Size, Polydispersity Index, and Zeta Potential
2.4. Determination of the Entrapment Efficiency and Loading Capacity
2.5. Morphological Characterizations
2.6. Determination of the Deformability Index
2.7. Stability of the Elastic Liposomes
2.8. Preparation of Porcine Buccal Tissues
2.9. Ex Vivo Permeability Studies
2.10. Localization Studies in Porcine Buccal Tissues
2.11. Statistical Analysis
3. Results
3.1. Physical Characterization of the Elastic Liposomes
3.2. Morphological Characterizations
3.3. Relative Deformability of Elastic Liposomes
3.4. Stability of the Elastic Liposomes
3.5. Permeability Studies across the Porcine Buccal Tissues
3.6. Localization Studies in the Porcine Buccal Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hua, S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, C.P.; Damgé, C. Nanotechnology as a Promising Strategy for Alternative Routes of Insulin Delivery. In Methods in Enzymology; Elsevier BV: Amsterdam, The Netherlands, 2012; Volume 508, pp. 271–294. [Google Scholar]
- Rathbone, M.J.; Drummond, B.K.; Tucker, I.G. The Oral Cavity as a Site for Systemic Drug Delivery. Adv. Drug Deliv. Rev. 1994, 13, 1–22. [Google Scholar] [CrossRef]
- Xiang, J.; Fang, X.; Li, X. Transbuccal Delivery of 2′, 3′-Dideoxycytidine: In Vitro Permeation Study and Histological Investiga-tion. Int. J. Pharm. 2002, 231, 57–66. [Google Scholar] [CrossRef]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and Revisited Approaches in Nanoparticle Systems for Buccal Drug Delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. An Overview of Polymeric Dosage Forms in Buccal Drug Delivery: State of Art, Design of Formulations and Their In Vivo Performance Evaluation. Mater. Sci. Eng. C 2018, 86, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barua, S.; Kim, H.; Jo, K.; Seo, C.W.; Park, T.J.; Bin Lee, K.; Yun, G.; Oh, K.; Lee, J. Drug Delivery Techniques for Buccal Route: Formulation Strategies and Recent Advances in Dosage Form Design. J. Pharm. Investig. 2016, 46, 593–613. [Google Scholar] [CrossRef]
- Morales, J.O.; McConville, J.T. Novel Strategies for the Buccal Delivery of Macromolecules. Drug Dev. Ind. Pharm. 2014, 40, 579–590. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Yang, H.M.; Kim, C.H.; Goo, Y.T.; Hwang, G.Y.; Chang, I.H.; Whang, Y.M.; Choi, Y.W. Enhanced Intracellular Delivery of BCG Cell Wall Skeleton into Bladder Cancer Cells Using Liposomes Functionalized with Folic Acid and Pep-1 Peptide. Pharmaceutics 2019, 11, 652. [Google Scholar] [CrossRef] [Green Version]
- Giri, B.; Kim, J.; Park, J.; Jin, S.; Kim, K.; Din, F.; Choi, H.-G.; Kim, D. Improved Bioavailability and High Photostability of Methotrexate by Spray-Dried Surface-Attached Solid Dispersion with an Aqueous Medium. Pharmaceutics 2021, 13, 111. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Xu, Y.; Meng, Y.; Zhang, X.; Xia, X.; Liu, Y. Factors Affecting the Buccal Delivery of Deformable Nanovesi-cles Based on Insulin–Phospholipid Complex: An In Vivo Investigation. Drug Deliv. 2020, 27, 900–908. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Wu, W. Liposomes Containing Glycocholate as Potential Oral Insulin Delivery Systems: Prepara-tion, In Vitro Characterization, and Improved Protection against Enzymatic Degradation. Int. J. Nanomed. 2011, 6, 1155–1166. [Google Scholar]
- Sonaje, K.; Tyagi, V.; Chen, Y.; Kalia, Y.N. Iontosomes: Electroresponsive Liposomes for Topical Iontophoretic Delivery of Chemotherapeutics to the Buccal Mucosa. Pharmaceutics 2021, 13, 88. [Google Scholar] [CrossRef]
- Bashyal, S.; Lee, S. Delivery of Biopharmaceuticals using Combination of Liposome and Iontophoresis: A Review. J. Pharm. Investig. 2015, 45, 611–624. [Google Scholar] [CrossRef]
- Khan, I.; Yousaf, S.; Najlah, M.; Ahmed, W.; Elhissi, A. Proliposome Powder or Tablets for Generating Inhalable Liposomes using a Medical Nebulizer. J. Pharm. Investig. 2021, 51, 61–73. [Google Scholar] [CrossRef]
- Yoon, S.-W.; Shin, D.H.; Kim, J.-S. Liposomal Itraconazole Formulation for the Treatment of Glioblastoma using Inclusion Com-plex with HP-β-CD. J. Pharm. Investig. 2019, 49, 477–483. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H. Nano-technology based Approaches for Anti-Diabetic Drugs Delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kim, C.H.; Yoon, H.Y.; Sung, S.W.; Goh, M.S.; Lee, E.S.; Shin, D.J.; Choi, Y.W. Steric Stabilization of RIPL Peptide-Conjugated Liposomes and In Vitro Assessment. J. Pharm. Investig. 2019, 49, 115–125. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel Vesicular Carriers for Enhanced Delivery: Char-acterization and Skin Penetration Properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Park, J.-B.; Noh, H.-G.; Jung, J.-H.; Kim, J.-M.; Kang, C.-Y. Enhanced Transdermal Delivery and Optimization of Nano-Liposome Preparation using Hydrophilic Drug. J. Pharm. Investig. 2012, 42, 57–63. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, Y.; Yang, Y.; Meng, Y.; Xia, X.; Liu, Y. Stabilization of Deformable Nanovesicles Based on Insulin-Phospholipid Complex by Freeze-Drying. Pharmaceutics 2019, 11, 539. [Google Scholar] [CrossRef] [Green Version]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a Tool for Transdermal and Dermal Delivery. Drug Discov. Today Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, A.; Oh, Y.-K.; Kim, C.-K. Effect of Edge Activators on the Formation and Transfection Efficiency of Ultrade-Formable Liposomes. Biomaterials 2005, 26, 205–210. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Revuri, V.; Nafiujjaman, M.; Cha, S.; Cho, S.; Huh, K.M.; Lee, Y.-K. Design and Strategies for Bile Acid Mediated Therapy and Imaging. RSC Adv. 2016, 6, 73986–74002. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Zhang, Y.; Ye, J.; Wang, H.-L.; Xia, X.; Liu, Y. Mechanisms of Deformable Nanovesicles Based on Insulin-phospholipid Complex for Enhancing Buccal Delivery of Insulin. Int. J. Nanomed. 2018, 13, 7319–7331. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-Z.; Wang, X.-T.; Yan, X.-Y.; Zhang, Q. Phospholipid Deformable Vesicles for Buccal Delivery of Insulin. Chem. Pharm. Bull. 2002, 50, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Choi, Y.W.; Lee, S. Facilitated Permeation of Insulin Across TR146 Cells by Cholic Acid Derivatives-Modified Elastic Bilosomes. Int. J. Nanomed. 2018, 13, 5173–5186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, W.; Hu, Q.; Xu, Y.; Xu, Y. Effect of Soybean-Lecithin as an Enhancer of Buccal Mucosa Absorption of Insulin. BioMed Mater. Eng. 2012, 22, 171–178. [Google Scholar] [CrossRef]
- Oh, D.-H.; Chun, K.-H.; Jeon, S.-O.; Kang, J.-W.; Lee, S. Enhanced Transbuccal Salmon Calcitonin (sCT) Delivery: Effect of Chemical Enhancers and Electrical Assistance on In Vitro sCT Buccal Permeation. Eur. J. Pharm. Biopharm. 2011, 79, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, S.; Shin, C.Y.; Hyun, S.M.; Jang, S.W.; Lee, S. Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl. Pharmaceutics 2020, 12, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. High Frequency Ultrasound Mediated Transdermal Delivery of On-dansetron Hydrochloride Employing Bilosomal Gel Systems: Ex-vivo and In-Vivo Characterization Studies. J. Pharm. Investig. 2020, 50, 613–624. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of Edge Activators and Surface Charge in Developing Ul-tradeformable Vesicles with Enhanced Skin Delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A Novel Vesicular Carrier for Enhanced Transdermal Delivery: Development, Characterization, and Performance Evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Kiio, T.M.; Park, S. Physical Properties of Nanoparticles Do Matter. J. Pharm. Investig. 2021, 51, 35–51. [Google Scholar] [CrossRef]
- Jeon, S.-O.; Hwang, H.-J.; Oh, D.-H.; Seo, J.-E.; Chun, K.-H.; Hong, S.-M.; Kim, M.-J.; Kim, W.-C.; Park, M.-S.; Yoon, C.-H.; et al. Enhanced Percutaneous Delivery of Recombinant Human Epidermal Growth Factor Employing Nano-Liposome System. J. Microencapsul. 2012, 29, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-J.; Karn, P.R.; Jin, S.-E.; Lee, B.J.; Kim, M.-S.; Sung, J.-H.; Sun, B.K. Preparation and Evaluation of Cyclosporin A-Containing Proliposomes: A Comparison of the Supercritical Antisolvent Process with the Conventional Film Method. Int. J. Nanomed. 2014, 9, 5079–5091. [Google Scholar] [CrossRef] [Green Version]
- Okafor, N.I.; Nkanga, C.I.; Walker, R.B.; Noundou, X.S.; Krause, R.W.M. Encapsulation and Physicochemical Evaluation of Efavirenz in Liposomes. J. Pharm. Investig. 2019, 50, 201–208. [Google Scholar] [CrossRef]
- Cui, M.; Wu, W.; Hovgaard, L.; Lu, Y.; Chen, D.; Qi, J. Liposomes Containing Cholesterol Analogues of Botanical Origin as Drug Delivery Systems to Enhance the Oral Absorption of Insulin. Int. J. Pharm. 2015, 489, 277–284. [Google Scholar] [CrossRef]
- Guan, P.; Lu, Y.; Qi, J.; Niu, M.; Lian, R.; Hu, F.; Wu, W. Enhanced Oral Bioavailability of Cyclosporine A by Liposomes Con-taining a Bile Salt. Int. J. Nanomed. 2011, 6, 965–974. [Google Scholar]
- Li, Z.; Zhang, M.; Liu, C.; Zhou, S.; Zhang, W.; Wang, T.; Zhou, M.; Liu, X.; Wang, Y.; Sun, Y.; et al. Development of Liposome Containing Sodium Deoxycholate to Enhance Oral Bioavailability of Itraconazole. Asian J. Pharm. Sci. 2017, 12, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Dai, Y.; Zhou, R.; Lu, Y.; Wu, W.; Liu, L. Liposomes Containing Bile Salts as Novel Ocular Delivery Systems for Tacrolimus (FK506): In Vitro Characterization and Improved Corneal Permeation. Int. J. Nanomed. 2013, 8, 1921–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.J.; Eum, J.Y.; Jeong, M.S.; Choi, S.E.; Park, S.H.; Cho, H.I.; Cho, C.S.; Seo, S.J.; Lee, M.W.; Choi, Y.W. Facilitated Skin Permeation of Oregonin by Elastic Liposomal Formulations and Suppression of Atopic Dermatitis in NC/Nga Mice. Biol. Pharm. Bull. 2010, 33, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeb, A.; Cha, J.-H.; Noh, A.R.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; Shin, D.; Shah, F.A.; Majid, A.; Bae, O.-N. Neuropro-tective Effects of Carnosine-Loaded Elastic Liposomes in Cerebral Ischemia Rat Model. J. Pharm. Investig. 2020, 50, 373–381. [Google Scholar] [CrossRef]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [Green Version]
- Keum, T.; Noh, G.; Seo, J.-E.; Bashyal, S.; Lee, S. In Vitro and Ex Vivo Evaluation of Penetratin as a Non-invasive Permeation Enhancer in the Penetration of Salmon Calcitonin through TR146 Buccal Cells and Porcine Buccal Tissues. Pharmaceuticals 2020, 13, 408. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pan, H.; Duan, H.; Deng, W.; Zhang, F.; Yang, X.; Pan, W. Self-Assembled Liposome from Core-Sheath Chitosan-Based Fibres for Buccal Delivery of Carvedilol: Formulation, Characterization and In Vitro and Ex Vivo Buccal Absorption. J. Pharm. Pharmacol. 2020, 72, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Ravivarapu, H.; Redkar, S.; Li, X.; Jasti, B.R. Transbuccal Delivery of 5-Aza-2′-Deoxycytidine: Effects of Drug Concentration, Buffer Solution, and Bile Salts on Permeation. AAPS PharmSciTech 2007, 8, E28–E33. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Attributes | SC Hydrate | SGDC |
|---|---|---|
| Mol wt. (g/mol) | 448.58 | 471.61 |
| H-bond donor | 4 | 3 |
| H-bond acceptor | 6 | 5 |
| CMC at 20–25 °C (mM) | 9–15 | 2.1 |
| Chemical structure |  |  |
| Formulation | Relative Deformability Index |
|---|---|
| 5% STC liposomes | 1.00 ± 0.04 |
| SC-EL | 1.11 ± 0.02 * |
| SGDC-EL | 1.57 ± 0.05 ***, ### |
| Formulation | Js (ng·cm−2·h−1) | Kp ((cm/h) × 10−5) | Lag time (h) | ER |
|---|---|---|---|---|
| Insulin solution | 12.05 ± 0.70 | 1.32 ± 0.08 | 0.97 ± 0.14 | 1.00 |
| Insulin + SC | 14.85 ± 1.12 | 1.63 ± 0.12 | 0.77 ± 0.39 | 1.23 |
| Insulin + SGDC | 21.36 ± 4.53 | 2.35 ± 0.50 | 0.68 ± 0.48 | 1.77 |
| SC-EL | 14.13 ± 2.24 | 1.55 ± 0.25 | 0.80 ± 0.24 | 1.17 |
| SGDC-EL | 52.18 ± 9.63 ***, ###, $$$ | 5.73 ± 1.06 ***, ###, $$$ | 0.48 ± 0.24 | 4.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Lamichhane, S.; Lee, S. Development, Characterization, and Ex Vivo Assessment of Elastic Liposomes for Enhancing the Buccal Delivery of Insulin. Pharmaceutics 2021, 13, 565. https://doi.org/10.3390/pharmaceutics13040565
Bashyal S, Seo J-E, Keum T, Noh G, Lamichhane S, Lee S. Development, Characterization, and Ex Vivo Assessment of Elastic Liposomes for Enhancing the Buccal Delivery of Insulin. Pharmaceutics. 2021; 13(4):565. https://doi.org/10.3390/pharmaceutics13040565
Chicago/Turabian StyleBashyal, Santosh, Jo-Eun Seo, Taekwang Keum, Gyubin Noh, Shrawani Lamichhane, and Sangkil Lee. 2021. "Development, Characterization, and Ex Vivo Assessment of Elastic Liposomes for Enhancing the Buccal Delivery of Insulin" Pharmaceutics 13, no. 4: 565. https://doi.org/10.3390/pharmaceutics13040565