Improving the Solubility, Dissolution, and Bioavailability of Metronidazole via Cocrystallization with Ethyl Gallate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Cocrystal
2.2.2. Preparation of Single Crystals
2.2.3. Single Crystal X-ray Diffraction (SCXRD)
2.2.4. Powder X-ray Diffraction (PXRD)
2.2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.2.6. Differential Scanning Calorimetry (DSC) and Thermogravimetry Analysis (TGA)
2.2.7. Scanning Electron Microscopy (SEM)
2.2.8. Powder Dissolution and Intrinsic Dissolution Rate (IDR)
2.2.9. Standard Curve for Determining Plasma Concentration of MTZ
2.2.10. Pharmacokinetic Study in Rats
3. Results and Discussion
3.1. PXRD Analysis
3.2. Crystal Structure
3.3. Fourier Transform Infrared (FT-IR) Spectroscopy
3.4. Thermal Analyses
3.5. Scanning Electron Microscopy (SEM)
3.6. Powder Dissolution and Intrinsic Dissolution Rate
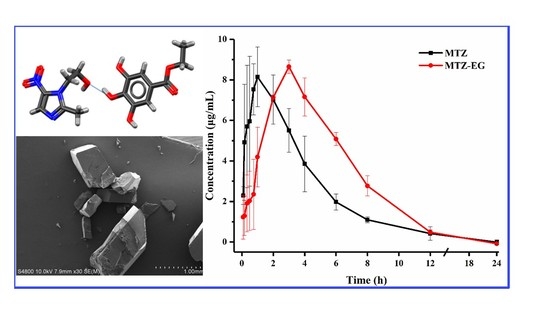
3.7. Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobel, R.; Sobel, J.D. Metronidazole for the Treatment of Vaginal Infections. Expert Opin. Pharmacother. 2015, 16, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.; Salazar, H.; Aoudjit, L.; Goncalves, R.; Zioui, D.; Fidalgo-Marijuan, A.; Costa, C.M.; Ferdov, S.; Lanceros-Mendez, S. Crystal Morphology Control of Synthetic Giniite for Enhanced Photo-Fenton Activity Against the Emerging Pollutant Metronidazole. Chemosphere 2021, 262, 128300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Li, A.; Wu, W.W.; Qian, S.S.; Liu, B.H.; Pang, Q.X. Preparation, Characterization, in Vitro and in Vivo Evaluation of Metronidazole–Gallic Acid Cocrystal: A Combined Experimental and Theoretical Investigation. J. Mol. Struct. 2019, 1197, 727–735. [Google Scholar] [CrossRef]
- Flemming, T.F.; Milian, E.; Kopp, C.; Ksrch, H.; Klaiber, B. Differential Effects of Systemic Metronidazole and Amoxicillin on Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis in Intraoral Habitats. J. Clin. Periodontol. 1998, 25, 1–10. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Rubini, D.; Giovagnoli, S.; Ricci, M.; Blasi, P.; Rossi, C. Novel Mucoadhesive Buccal Formulation Containing Metronidazole for the Treatment of Periodontal Disease. J. Control. Releas 2004, 95, 521–533. [Google Scholar] [CrossRef]
- Mohanty, K.; Deighton, R. Comparison of Two Different Metronidazole Regimens in the Treatment of Gardnerella Vaginalis Infection with or Without Trichomoniasis. J. Antimicrob. Chemother. 1985, 16, 799–803. [Google Scholar] [CrossRef]
- Andrade, F.; Rafael, D.; Videira, M.; Ferreira, D.; Sosnik, A.; Sarmento, B. Nanotechnology and Pulmonary Delivery to Overcome Resistance in Infectious Diseases. Adv. Drug Del. Rev. 2013, 65, 1816–1827. [Google Scholar] [CrossRef]
- Gordon, A.T.; Abosede, O.O.; Ntsimango, S.; Vuuren, S.V.; Hosten, E.C.; Ogunlaja, A.S. Synthesis, Characterization, Molecular Docking and Antimicrobial Activity of Copper(II) Complexes of Metronidazole and 1,10 Phenanthroline. Inorg. Chim. Acta. 2020, 510, 119744. [Google Scholar] [CrossRef]
- Li, H.-Q.; Xiao, Z.-P.; Fang, R.-Q.; Zhu, H.-L. The Syntheses and Crystal Structures of Metronidazole-derived Compounds. J. Chem. Crystallogr. 2008, 38, 461–466. [Google Scholar] [CrossRef]
- Sekis, I.; Ramstead, K.; Rishniw, M.; Schwark, W.S.; McDonough, S.P.; Goldstein, R.E.; Papich, M.; Simpson, K.W. Single-Dose Pharmacokinetics and Genotoxicity of Metronidazole in Cats. J. Feline Med. Surg. 2009, 11, 60–68. [Google Scholar] [CrossRef]
- Fenimore, A.; Martin, L.; Lappin, M.R. Evaluation of Metronidazole with and Without Enterococcus Faecium SF68 in Shelter Dogs With Diarrhea. Top. Companion Anim. Med. 2017, 32, 100–103. [Google Scholar] [CrossRef]
- Hui, L.; Xiaoxiang, Z.; Shaomin, Z. Pharmacokinetics of Metronidazole Colon-Targeted Capsules in Dogs. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, iCBBE, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Zarekar, A.U.; Markandeya, N.M.; Bhalerao, D.P. Enrofloxacin and Metronidazole Combination Therapy for Management of Infectious Repeat Breeder Syndrome in Crossbred cows. Intas. Polivet. 2019, 20, 20–24. [Google Scholar]
- Stein, F.; Gilliam, L.; Davis, J.; Taylor, J. Rectal Administration of Metronidazole with and Without Rectal Evacuation Prior to Use in Horses. J. Vet. Pharmacol. Ther. 2018, 41, 838–842. [Google Scholar] [CrossRef]
- Lin, Y.; Su, Y.; Liao, X.; Yang, N.; Yang, X.; Choi, M.M. Determination of Five Nitroimidazole Residues in Artificial Porcine Muscle Tissue Samples by Capillary Electrophoresis. Talanta 2012, 88, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Gentili, A.; Perret, D.; Marchese, S. Liquid Chromatography-Tandem Mass Spectrometry for Performing Confirmatory Analysis of Veterinary Drugs in Animal-Food Products. TrAC Trends Anal. Chem. 2005, 24, 704–733. [Google Scholar] [CrossRef]
- Stjern, L.; Voittonen, S.; Weldemichel, R.; Thuresson, S.; Agnes, M.; Benkovics, G.; Fenyvesi, E.; Malanga, M.; Yannakopoulou, K.; Feiler, A.; et al. Cyclodextrin-Mesoporous Silica Particle Composites for Controlled Antibiotic Release. A Proof of Concept Toward Colon Targeting. Int. J. Pharm. 2017, 531, 595–605. [Google Scholar] [CrossRef]
- Rocha-Garduno, G.; Hernandez-Martinez, N.A.; Colin-Lozano, B.; Estrada-Soto, S.; Hernandez-Nunez, E.; Prieto-Martinez, F.D.; Medina-Franco, J.L.; Chale-Dzul, J.B.; Moo-Puc, R.; Navarrete-Vazquez, G. Metronidazole and Secnidazole Carbamates: Synthesis, Antiprotozoal Activity, and Molecular Dynamics Studies. Molecules 2020, 25, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.E.; Elasala, G.S.; Ibrahim, R.S. Synthesis, Characterization, Spectral, Thermal Analysis and Biological Activity Studies of Metronidazole Complexes. J. Mol. Struct. 2019, 1176, 673–684. [Google Scholar] [CrossRef]
- Waszczykowska, A.; Żyro, D.; Jurowski, P.; Ochocki, J. Effect of Treatment with Silver(I) Complex of Metronidazole on Ocular Rosacea: Design and Formulation of New Silver Drug with Potent Antimicrobial Activity. J. Trace Elem. Med. Biol. 2020, 61. [Google Scholar] [CrossRef]
- Sabbagh, H.A.K.; Abudayeh, Z.; Abudoleh, S.M.; Alkrad, J.A.; Hussein, M.Z.; Hussein-Al-Ali, S.H. Application of Multiple Regression Analysis in Optimization of Metronidazole-Chitosan Nanoparticles. J. Polym. Res. 2019, 26, 205. [Google Scholar] [CrossRef]
- Sabbagh, H.A.K.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Abudayeh, Z.; Ayoub, R.; Abudoleh, S.M. A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design. Polymers 2020, 12, 772. [Google Scholar] [CrossRef] [Green Version]
- Leber, A.; Budai-Szucs, M.; Urban, E.; Valyi, P.; Gacsi, A.; Berko, S.; Kovacs, A.; Csanyi, E. Combination of Zinc Hyaluronate and Metronidazole in a Lipid-Based Drug Delivery System for the Treatment of Periodontitis. Pharmaceutics 2019, 11, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, A.; Uyar, T. Metronidazole/Hydroxypropyl-beta-Cyclodextrin Inclusion Complex Nanofibrous Webs as Fast-Dissolving Oral Drug Delivery System. Int. J. Pharm. 2019, 572, 118828. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, A.; Agarwal, S.M.; Avecilla, F.; Azam, A. Metronidazole Thiosalicylate Conjugates: Synthesis, Crystal Structure, Docking Studies and Antiamoebic Activity. Bioorg. Med. Chem. Lett. 2012, 22, 5694–5699. [Google Scholar] [CrossRef]
- Li, W.; Pi, J.; Zhang, Y.; Ma, X.; Zhang, B.; Wang, S.; Qi, D.; Li, N.; Guo, P.; Liu, Z. A Strategy to Improve the Oral Availability of Baicalein: The Baicalein-Theophylline Cocrystal. Fitoterapia 2018, 129, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Cheney, M.L.; Weyna, D.R.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Coformer Selection in Pharmaceutical Cocrystal Development: A Case Study of a Meloxicam Aspirin Cocrystal that Exhibits Enhanced Solubility and Pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, S.; Mannava, M.K.C.; Khandavilli, U.B.R.; Allu, S.; Nangia, A. Soluble Salts and Cocrystals of Clotrimazole. Cryst. Growth Des. 2015, 15, 2493–2504. [Google Scholar] [CrossRef]
- Aitipamula, S.; Vangala, V.R.; Chow, P.S.; Tan, R.B.H. Cocrystal Hydrate of an Antifungal Drug, Griseofulvin, with Promising Physicochemical Properties. Cryst. Growth Des. 2012, 12, 5858–5863. [Google Scholar] [CrossRef]
- Nugrahani, I.; Kumalasari, R.A.; Auli, W.N.; Horikawa, A.; Uekusa, H. Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics 2020, 12, 690. [Google Scholar] [CrossRef]
- Surov, A.O.; Manin, A.N.; Voronin, A.P.; Drozd, K.V.; Simagina, A.A.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical Salts of Ciprofloxacin with Dicarboxylic Acids. Eur. J. Pharm. Sci. 2015, 77, 112–121. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Pharmaceutical Cocrystals of Ethenzamide: Structural, Solubility and Dissolution Studies. CrystEngComm 2012, 14, 8515–8524. [Google Scholar] [CrossRef]
- Yamashita, H.; Sun, C.C. Improving Dissolution Rate of Carbamazepine-Glutaric Acid Cocrystal Through Solubilization by Excess Coformer. Pharm. Res. 2017, 35, 4. [Google Scholar] [CrossRef] [PubMed]
- Bofill, L.; de Sande, D.; Barbas, R.; Prohens, R. New Cocrystal of Ubiquinol with High Stability to Oxidation. Cryst. Growth Des. 2020, 20, 5583–5588. [Google Scholar] [CrossRef]
- Sun, C.C.; Hou, H. Improving Mechanical Properties of Caffeine and Methyl Gallate Crystals by Cocrystallization. Cryst. Growth Des. 2008, 8, 1575–1579. [Google Scholar] [CrossRef] [Green Version]
- Krishna, G.R.; Shi, L.; Bag, P.P.; Sun, C.C.; Reddy, C.M. Correlation among Crystal Structure, Mechanical Behavior, and Tabletability in the Co-Crystals of Vanillin Isomers. Cryst. Growth Des. 2015, 15, 1827–1832. [Google Scholar] [CrossRef]
- Zheng, K.; Gao, S.; Chen, M.; Li, A.; Wu, W.; Qian, S.; Pang, Q. Color Tuning of an Active Pharmaceutical Ingredient Through Cocrystallization: A Case Study of a Metronidazole–Pyrogallol Cocrystal. CrystEngComm 2020, 22, 1404–1413. [Google Scholar] [CrossRef]
- Qu, H.; Pan, L.; Sun, Y.; Wang, L.; Li, Y.; Zhang, M.; Zhang, Z.; Lin, H. Supramolecular Assemblies of Three New Metronidazole Derivatives Constructed with Various Dihydroxy-benzoic Acids via Hydrogen Bonds. Chem. Res. Chin. Univ. 2020, 36, 1196–1202. [Google Scholar] [CrossRef]
- Seera, R.; Guru Row, T.N. Evaluation of Cocrystallization Outcomes of Multicomponent Adducts: Rapid Fabrication to Achieve Uniform Particle Size Distribution Using Thermal Inkjet Printing. Cryst. Growth Des. 2020, 20, 4667–4677. [Google Scholar] [CrossRef]
- Cui, W.; He, Z.; Zhang, Y.; Fan, Q.; Feng, N. Naringenin Cocrystals Prepared by Solution Crystallization Method for Improving Bioavailability and Anti-Hyperlipidemia Effects. AAPS PharmSciTech 2019, 20, 115. [Google Scholar] [CrossRef]
- Okoye, E. Improvement of the Crystal Stability and Dissolution Profile of Metronidazole by Composite Formation with Microcrystalline Cellulose and Cashew Gum. J. Pharm. Allied Sci. 2014, 11, 2006–2026. [Google Scholar]
- Zhao, X.; Li, Q.; Wang, C.; Hu, S.; He, X.; Sun, C.C. Simultaneous Taste-Masking and Oral Bioavailability Enhancement of Ligustrazine by Forming Sweet Salts. Int. J. Pharm. 2020, 577, 119089. [Google Scholar] [CrossRef]









| Formula | C15H19N3O8 |
|---|---|
| CCDC no. | 2,063,084 |
| Formula weight | 369.33 |
| T/K | 200 |
| Wavelength/Å | 1.54184 |
| Crystal system | Monoclinic |
| Space group | I 2/a |
| a/Å | 16.5805 (7) |
| b/Å | 13.2924 (4) |
| c/Å | 16.5248 (6) |
| α/° | 90 |
| β/° | 106.018 (4) |
| γ/° | 90 |
| V/Å3 | 3500.6 (2) |
| Z | 8 |
| Dx/g cm−3 | 1.402 |
| Rint | 0.048162 |
| Tmin | 0.536 |
| Tmax | 1 |
| h, k, lmax | 21, 16, 21 |
| Mu (mm−1) | 0.985 |
| F000 | 1552 |
| θmax | 79.654 |
| GooF | 1.056 |
| R1, wR2 [I ≥ 2σ(I)]a | 0.0878, 0.2616 |
| R1,wR2 [all data]b | 0.0947, 0.2648 |
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | D-H···A (°) |
|---|---|---|---|---|
| O6-H6···O7 i | 0.82 | 1.92 | 2.708 (3) | 161.5 |
| O5-H5···N2 ii | 0.82 | 1.96 | 2.781 (3) | 178.1 |
| O3-H3···O4 iii | 0.82 | 2.38 | 3.037 (2) | 137.8 |
| T1/2 (min) | Tmax (min) | Cmax (μg/mL) | AUC0–24 (μg/mL·min) | |
|---|---|---|---|---|
| MTZ | 106.95 ± 7.29 | 55.00 ± 8.66 | 8.15 ± 1.46 | 2345.98 ± 409.63 |
| MTZ-EG | 100.82 ± 15.98 | 180.00 ± 0.00 | 8.64 ± 0.33 | 3181.84 ± 212.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hao, X.; Wang, C.; Liu, H.; Liu, L.; He, X.; Sun, C.C. Improving the Solubility, Dissolution, and Bioavailability of Metronidazole via Cocrystallization with Ethyl Gallate. Pharmaceutics 2021, 13, 546. https://doi.org/10.3390/pharmaceutics13040546
Li J, Hao X, Wang C, Liu H, Liu L, He X, Sun CC. Improving the Solubility, Dissolution, and Bioavailability of Metronidazole via Cocrystallization with Ethyl Gallate. Pharmaceutics. 2021; 13(4):546. https://doi.org/10.3390/pharmaceutics13040546
Chicago/Turabian StyleLi, Jinhui, Xinghui Hao, Chenguang Wang, Haiyan Liu, Lianchao Liu, Xin He, and Changquan Calvin Sun. 2021. "Improving the Solubility, Dissolution, and Bioavailability of Metronidazole via Cocrystallization with Ethyl Gallate" Pharmaceutics 13, no. 4: 546. https://doi.org/10.3390/pharmaceutics13040546