Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants
Abstract
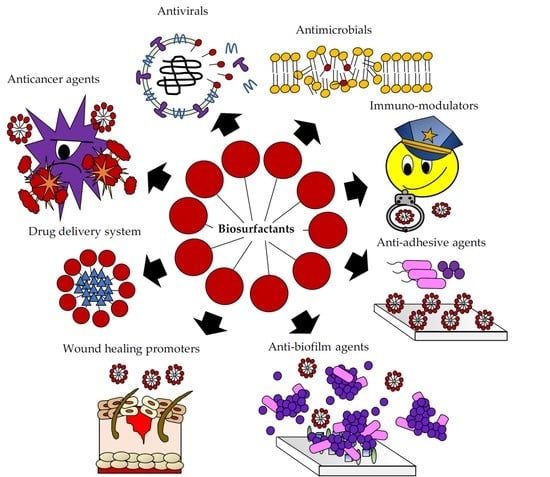
:1. Introduction
2. Biosurfactant Properties and Biological Activities Useful for Biomedical and Pharmaceutical Applications
3. Antimicrobial Activity of BSs
3.1. Lipopeptides and Glycolipids as Antimicrobial Agents
3.2. Biosurfactants from Lactic Acid Bacteria with Antimicrobial Activities
4. Antiadhesive and Antibiofilm Activity of BSs
4.1. Lipopeptides and Glycolipids as Antiadhesive/Antibiofilm Agents
4.2. Biosurfactants from Lactic Acid Bacteria with Antiadhesive/Antibiofilm Properties
4.3. Synergism of BSs with Other Molecules
5. Antiviral Activity
6. Wound Healing
7. Anticancer Activity of BSs
8. Immuno-Modulatory Activity of BSs
9. Commercial Applications in the Biomedical and Pharmaceutical Fields
10. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morita, T.; Ishibashi, Y.; Hirose, N.; Wada, K.; Takahashi, M.; Fukuoka, T.; Imura, T.; Sakai, H.; Abe, M.; Kitamoto, D. Production and characterization of a glycolipid biosurfactant, mannosylerythritol lipid B, from sugarcane juice by Ustilago scitaminea NBRC 32730. Biosci. Biotechnol. Biochem. 2011, 75, 1371–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Mixing behavior of the biosurfactant, rhamnolipid, with a conventional anionic surfactant, sodium dodecyl benzene sulfonate. Langmuir 2010, 26, 17958–17968. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Solution self-assembly and adsorption at the air-water interface of the monorhamnose and dirhamnose rhamnolipids and their mixtures. Langmuir 2010, 26, 18281–18292. [Google Scholar] [CrossRef]
- Martinotti, M.G.; Allegrone, G.; Cavallo, M.; Fracchia, L. Biosurfactants. In Innovative Technologies for Sustainable Development; Piemonte, V., De Falco, M., Basile, A., Eds.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Mandal, S.M.; Barbosa, A.E.A.D.; Franco, O.L. Lipopeptides in microbial infection control: Scope and reality for industry. Biotechnol. Adv. 2013, 31, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Van Hamme, J.D.; Ward, O.P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol. Adv. 2007, 25, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 2011, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracchia, L.; Ceresa, C.; Franzetti, A.; Cavallo, M.; Gandolfi, I.; Van Hamme, J.; Gkorezis, P.; Marchant, R.; Banat, I.M. Industrial Applications of Biosurfactants. In Biosurfactants: Production and Utilization-Processes, Technologies and Economics; Kosaric, N., Sukan, F.V., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 245–267. [Google Scholar]
- Swarnalatha, M.S.; Rani, J.C. Biosurfactants: Unique properties and their versatile applications. Pharma Innovat. J. 2019, 8, 684–687. [Google Scholar]
- Kłosowska-Chomiczewska, I.E.; Mędrzycka, K.; Karpenko, E. Biosurfactants–biodegradability, toxicity, efficiency in comparison with synthetic surfactants. Adv. Chem. Mech. Eng. 2011, 2, 1–9. [Google Scholar]
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Carvalho, A.M.X.; Tótola, M.R.; Borges, A.C. Biodegradability of bacterial surfactants. Biodegradation 2011, 22, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.B.; Mishra, A. Sustainable Biosurfactants. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–11. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; Cázares-Marinero, J.d.J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. Part A 2021, 320, 124222. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Homaei, A.; Patil, S.; Daverey, A. Microbial biosurfactants for oil spill remediation: Pitfalls and potentials. Appl. Microbiol. Biotechnol. 2019, 103, 27–37. [Google Scholar] [CrossRef]
- Decesaro, A.; Machado, T.S.; Cappellaro, Â.C.; Reinehr, C.O.; Thomé, A.; Colla, L.M. Biosurfactants during in situ bioremediation: Factors that influence the production and challenges in evalution. Environ. Sci. Pollut. Res. 2017, 24, 20831–20843. [Google Scholar] [CrossRef]
- Millioli, V.S.; Servulo, E.-L.C.; Sobral, L.G.S.; De Carvalho, D.D. Bioremediation of crude oil-bearing soil: Evaluating the effect of rhamnolipids addition to soil toxicity and to crude oil biodegradation efficiency. Glob. NEST J. 2009, 11, 181–188. [Google Scholar]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Biological surfactants vs. polysorbates: Comparison of their emulsifier and surfactant properties. Tenside Surfactants Deterg. 2018, 55, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Fracchia, L.; Banat, J.J.; Cavallo, M.; Ceresa, C.; Banat, I.M. Potential therapeutic applications of microbial surface-active compounds. AIMS Bioeng. 2015, 2, 144. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [Green Version]
- Fracchia, L.; Ceresa, C.; Banat, I.M. Biosurfactants in Cosmetic, Biomedical and Pharmaceutical Industry. In Microbial Biosurfactants and Their Environmental and Industrial Applications; Banat, I.M., Thavasi, R., Eds.; CRS Press: Boca Raton, FL, USA, 2019; pp. 258–288. [Google Scholar]
- Fracchia, L.; Cavallo, M.; Martinotti, M.G.; Banat, I.M. Biosurfactants and bioemulsifiers: Biomedical and related applications-present status and future potentials. In Biomedical Science, Engineering and Technology; Ghista, D.N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 325–370. [Google Scholar]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Physiological aspects Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, L.; Ławniczak, L.; Czaczyk, K. Why do microorganisms produce rhamnolipids? World J. Microbiol. Biotechnol. 2012, 28, 401–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamp, S.J.; Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [Green Version]
- Satpute, S.K.; Banpurkar, A.G.; Banat, I.M.; Sangshetti, J.N.; Patil, R.H.; Gade, W.N. Multiple Roles of Biosurfactants in Biofilms. Curr. Pharm. Des. 2016, 22, 1429–1448. [Google Scholar] [CrossRef] [Green Version]
- Horn, J.N.; Sengillo, J.D.; Lin, D.; Romo, T.D.; Grossfield, A. Characterization of a potent antimicrobial lipopeptide via coarse-grained molecular dynamics. Biochim. Biophys. Acta 2012, 1818, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Cortés-Sánchez, A.J.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef]
- Walencka, E.; Rozalska, S.; Sadowska, B.; Rozalska, B. The Influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008, 53, 61–66. [Google Scholar] [CrossRef]
- La Fauci, V.; Alessi, V. Antibiotic resistance: Where are we going? Ann. Ig. 2018, 30, 52–57. [Google Scholar]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Robbel, L.; Marahiel, M.A. Daptomycin, a bacterial lipopeptide synthesized by a nonribosomal machinery. J. Biol. Chem. 2010, 285, 27501–27508. [Google Scholar] [CrossRef] [Green Version]
- Ngai, A.L.; Bourque, M.R.; Lupinacci, R.J.; Strohmaier, K.M.; Kartsonis, N.A. Overview of safety experience with caspofungin in clinical trials conducted over the first 15 years: A brief report. Int. J. Antimicrob. Agents 2011, 38, 540–544. [Google Scholar] [CrossRef]
- Emiroglu, M. Micafungin use in children. Expert Rev. Anti Infect. Ther. 2011, 9, 821–834. [Google Scholar] [CrossRef]
- George, J.; Reboli, A.C. Anidulafungin: When and how? The clinician’s view. Mycoses 2012, 55, 36–44. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus spp.: A Gold Mine of Antibiotic Candidates. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef] [Green Version]
- Vater, J.; Kablitz, B.; Wilde, C.; Franke, P.; Mehta, N.; Cameotra, S.S. Matrix-assisted laser desorption ionization--time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 2002, 68, 6210–6219. [Google Scholar] [CrossRef] [Green Version]
- Naruse, N.; Tenmyo, O.; Kobaru, S.; Kamei, H.; Miyaki, T.; Konishi, M.; Oki, T. Pumilacidin, a complex of new antiviral antibiotics. Production, isolation, chemical properties, structure and biological activity. J. Antibiot. 1990, 43, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Grangemard, I.; Wallach, J.; Maget-Dana, R.; Peypoux, F. Lichenysin: A more efficient cation chelator than surfactin. Appl. Biochem. Biotechnol. 2001, 90, 199–210. [Google Scholar] [CrossRef]
- Saini, H.S.; Barragán-Huerta, B.E.; Lebrón-Paler, A.; Pemberton, J.E.; Vázquez, R.R.; Burns, A.M.; Marron, M.T.; Seliga, C.J.; Gunatilaka, A.A.L.; Maier, R.M. Efficient purification of the biosurfactant viscosin from Pseudomonas libanensis strain M9-3 and its physicochemical and biological properties. J. Nat. Prod. 2008, 71, 1011–1015. [Google Scholar] [CrossRef]
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 2004, 85, 1–8. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, D.; Yanagishita, H.; Shinbo, T.; Nakane, T.; Kamisawa, C.; Nakahara, T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J. Biotechnol. 1993, 29, 91–96. [Google Scholar] [CrossRef]
- Yang, X.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation and Structural Elucidation of Brevibacillin, an Antimicrobial Lipopeptide from Brevibacillus laterosporus That Combats Drug-Resistant Gram-Positive Bacteria. Appl. Environ. Microbiol. 2016, 82, 2763–2772. [Google Scholar] [CrossRef] [Green Version]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Mnif, I.; Kammoun, R.; Ayadi, I.; Saadaoui, I.; Maktouf, S.; Chaabouni-Ellouze, S. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J. Biomed. Biotechnol. 2012, 2012, 373682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mnif, I.; Grau-Campistany, A.; Coronel-León, J.; Hammami, I.; Triki, M.A.; Manresa, A.; Ghribi, D. Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani. Environ. Sci. Pollut. Res. Int. 2016, 23, 6690–6699. [Google Scholar] [CrossRef]
- Bouassida, M.; Fourati, N.; Krichen, F.; Zouari, R.; Ellouz-Chaabouni, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 lipopeptides in toothpaste formulation. J. Adv. Res. 2017, 8, 425–433. [Google Scholar] [CrossRef]
- Cordeiro, R.d.A.; Weslley Caracas Cedro, E.; Raquel Colares Andrade, A.; Serpa, R.; José de Jesus Evangelista, A.; Sales de Oliveira, J.; Santos Pereira, V.; Pereira Alencar, L.; Bruna Leite Mendes, P.; Cibelle Soares Farias, B.; et al. Inhibitory effect of a lipopeptide biosurfactant produced by Bacillus subtilis on planktonic and sessile cells of Trichosporon spp. Biofouling 2018, 34, 309–319. [Google Scholar] [CrossRef]
- Basit, M.; Rasool, M.H.; Naqvi, S.A.R.; Waseem, M.; Aslam, B. Biosurfactants production potential of native strains of Bacillus cereus and their antimicrobial, cytotoxic and antioxidant activities. Pak. J. Pharm. Sci. 2018, 31, 251–256. [Google Scholar]
- Medeot, D.B.; Fernandez, M.; Morales, G.M.; Jofré, E. Fengycins From Bacillus amyloliquefaciens MEP218 Exhibit Antibacterial Activity by Producing Alterations on the Cell Surface of the Pathogens Xanthomonas axonopodis Pv. vesicatoria and Pseudomonas aeruginosa PA01. Front. Microbiol. 2020, 10, 3107. [Google Scholar] [CrossRef]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 2017, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Mani, P.; Dineshkumar, G.; Jayaseelan, T.; Deepalakshmi, K.; Ganesh Kumar, C.; Senthil Balan, S. Antimicrobial activities of a promising glycolipid biosurfactant from a novel marine Staphylococcus saprophyticus SBPS 15. 3 Biotech 2016, 6, 163. [Google Scholar] [CrossRef] [Green Version]
- Valotteau, C.; Banat, I.M.; Mitchell, C.A.; Lydon, H.; Marchant, R.; Babonneau, F.; Pradier, C.-M.; Baccile, N.; Humblot, V. Antibacterial properties of sophorolipid-modified gold surfaces against Gram positive and Gram negative pathogens. Colloids Surf. B Biointerfaces 2017, 157, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef]
- Elshikh, M.; Moya-Ramírez, I.; Moens, H.; Roelants, S.; Soetaert, W.; Marchant, R.; Banat, I.M. Rhamnolipids and lactonic sophorolipids: Natural antimicrobial surfactants for oral hygiene. J. Appl. Microbiol. 2017, 123, 1111–1123. [Google Scholar] [CrossRef]
- Sen, S.; Borah, S.N.; Kandimalla, R.; Bora, A.; Deka, S. Efficacy of a rhamnolipid biosurfactant to inhibit Trichophyton rubrum in vitro and in a mice model of dermatophytosis. Exp. Dermatol. 2019, 28, 601–608. [Google Scholar] [CrossRef]
- Khan, F.; Oloketuyi, S.F.; Kim, Y.-M. Diversity of bacteria and bacterial products as antibiofilm and antiquorum sensing drugs against pathogenic bacteria. J. Hazard. Mater. 2019, 364, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, Anti-Adhesive and Anti-Biofilm potential of Biosurfactants Isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathogen. 2019, 127, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Karczewska, M.; Myszka, K.; Sznajdrowska, A.; Szulc, A.; Zgoła-Grześkowiak, A.; Ławniczak, Ł.; Corvini, P.F.-X.; Chrzanowski, Ł. Isolation of rhamnolipids-producing cultures from faeces: Influence of interspecies communication on the yield of rhamnolipid congeners. New Biotechnol. 2017, 36, 17–25. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Fernandes, E.C.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and anti-adhesive activities of cell-bound biosurfactant from Lactobacillus agilis CCUG31450. RSC Adv. 2015, 5, 90960–90968. [Google Scholar] [CrossRef] [Green Version]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef]
- Fariq, A.; Saeed, A. Production and Biomedical Applications of Probiotic Biosurfactants. Curr. Microbiol. 2016, 72, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb. Cell Fact. 2017, 16, 155. [Google Scholar] [CrossRef]
- Vecino, X.; Rodríguez-López, L.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Bioactivity of glycolipopeptide cell-bound biosurfactants against skin pathogens. Int. J. Biol. Macromol. 2018, 109, 971–979. [Google Scholar] [CrossRef]
- Ghasemi, A.; Moosavi-Nasab, M.; Setoodeh, P.; Mesbahi, G.; Yousefi, G. Biosurfactant Production by Lactic Acid Bacterium Pediococcus dextrinicus SHU1593 Grown on different carbon sources: Strain screening followed by product characterization. Sci. Rep. 2019, 9, 5287. [Google Scholar] [CrossRef] [Green Version]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Fact. 2016, 15, 165. [Google Scholar] [CrossRef] [Green Version]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, E59. [Google Scholar] [CrossRef] [Green Version]
- Burmølle, M.; Ren, D.; Bjarnsholt, T.; Sørensen, S.J. Interactions in multispecies biofilms: Do they actually matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Desvaux, M.; Hébraud, M.; Møretrø, T.; Langsrud, S.; Doulgeraki, A.; Nychas, G.-J.; Kačániová, M.; Czaczyk, K.; et al. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front. Microbiol. 2015, 6, 841. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial biofilms in urinary tract infections and prostatitis: Etiology, pathogenicity, and combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Pompilio, A.; Bonaventura, G.D. Microbial biofilm: A “sticky” problem. Microbiol. Med. 2018, 33, 7851. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalheiro, M.; Teixeira, M.C. Candida biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid. Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dragoš, A.; Kovács, Á.T. The peculiar functions of the bacterial extracellular matrix. Trends Microbiol. 2017, 25, 257–266. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef]
- Monds, R.D.; O’Toole, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2009, 17, 73–87. [Google Scholar] [CrossRef]
- Petrova, O.E.; Sauer, K. Escaping the biofilm in more than one way: Desorption, detachment or dispersion. Curr. Opin. Microbiol. 2016, 30, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—an overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Rumbaugh, K.P. Approaches to dispersing medical biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Rivardo, F.; Martinotti, M.G.; Turner, R.J.; Ceri, H. Synergistic effect of lipopeptide biosurfactant with antibiotics against Escherichia coli CFT073 biofilm. Int. J. Antimicrob. Agents 2011, 37, 324–331. [Google Scholar] [CrossRef]
- Joshi-Navare, K.; Prabhune, A. A biosurfactant-sophorolipid acts in synergy with antibiotics to enhance their efficiency. BioMed. Res. Int. 2013, 2013, 512495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceresa, C.; Rinaldi, M.; Fracchia, L. Synergistic activity of antifungal drugs and lipopeptide AC7 against Candida albicans biofilm on silicone. AIMS Bioeng. 2017, 4, 318. [Google Scholar] [CrossRef]
- Banat, I.M.; Díaz De Rienzo, M.A.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Moryl, M.; Płaza, G.; Bhagat, D.; Satpute, S.K.; Bernat, P. Surfactants of microbial origin as antibiofilm agents. Int. J. Environ. Health Res. 2019, 1–20. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Tessarolo, F.; Maniglio, D.; Fedeli, E.; Tambone, E.; Caciagli, P.; Banat, I.M.; Diaz De Rienzo, M.A.; Fracchia, L. Inhibitory effects of lipopeptides and glycolipids on C. albicans–Staphylococcus spp. Dual-Species Biofilms. Front. Microbiol. 2021, 11, 545654. [Google Scholar] [CrossRef]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005, 57, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Ibacache-Quiroga, C.; Ojeda, J.; Espinoza-Vergara, G.; Olivero, P.; Cuellar, M.; Dinamarca, M.A. The hydrocarbon-degrading marine bacterium Cobetia sp. strain MM1IDA2H-1 produces a biosurfactant that interferes with quorum sensing of fish pathogens by signal hijacking. Microb. Biotechnol. 2013, 6, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Sci. Rep. 2016, 6, 23575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, L.R.; Banat, I.M.; van der Mei, H.C.; Teixeira, J.A.; Oliveira, R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J. Appl. Microbiol. 2006, 100, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Quinn, G.A.; Maloy, A.P.; Banat, M.M.; Banat, I.M. A comparison of effects of broad-spectrum antibiotics and biosurfactants on established bacterial biofilms. Curr. Microbiol. 2013, 67, 614–623. [Google Scholar] [CrossRef]
- Sharma, D.; Saharan, B.S. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol. Rep. 2016, 11, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Ciandrini, E.; Campana, R.; Casettari, L.; Perinelli, D.R.; Fagioli, L.; Manti, A.; Palmieri, G.F.; Papa, S.; Baffone, W. Characterization of biosurfactants produced by Lactobacillus spp. and their activity against oral streptococci biofilm. Appl. Microbiol. Biotechnol. 2016, 100, 6767–6777. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, I.; Petkovic, M.; Jovanovic, M.; Milivojevic, D.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Anti-biofilm properties of bacterial di-rhamnolipids and their semi-synthetic amide derivatives. Front. Microbiol. 2017, 8, 2454. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Zhu, X.; Zhao, H.; Lu, Y.; Zhang, C.; Lu, Z. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl. Microbiol. Biotechnol. 2019, 103, 4565–4574. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, F.; Jardak, M.; Elloumi, J.; Stien, D.; Cherif, S.; Mnif, S.; Aifa, S. Antibacterial, anti-adherent and cytotoxic activities of surfactin(s) from a lipolytic strain Bacillus safensis F4. Biodegradation 2019, 30, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Ryu, E.C.; Sukumaran, V.; Park, S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-grade silicone coated with rhamnolipid R89 is Effective against Staphylococcus spp. biofilms. Molecules 2019, 24, 3843. [Google Scholar] [CrossRef] [Green Version]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; Díaz De Rienzo, M.A. The effect of sophorolipids against microbial biofilms on medical-grade silicone. J. Biotechnol. 2020, 309, 34–43. [Google Scholar] [CrossRef]
- Ceresa, C.; Hutton, S.; Lajarin-Cuesta, M.; Heaton, R.; Hargreaves, I.; Fracchia, L.; Díaz De Rienzo, M.A. Production of Mannosylerythritol Lipids (MELs) to be used as antimicrobial agents against S. aureus ATCC 6538. Curr. Microbiol. 2020, 77, 1373–1380. [Google Scholar] [CrossRef] [Green Version]
- Janek, T.; Krasowska, A.; Czyżnikowska, Ż.; Łukaszewicz, M. Trehalose Lipid biosurfactant reduces adhesion of microbial pathogens to polystyrene and silicone surfaces: An experimental and computational approach. Front. Microbiol. 2018, 9, 2441. [Google Scholar] [CrossRef] [Green Version]
- Satpute, S.K.; Mone, N.S.; Das, P.; Banpurkar, A.G.; Banat, I.M. Lactobacillus acidophilus derived biosurfactant as a biofilm inhibitor: A promising investigation using microfluidic approach. Appl. Sci. 2018, 8, 1555. [Google Scholar] [CrossRef] [Green Version]
- Satpute, S.K.; Mone, N.S.; Das, P.; Banat, I.M.; Banpurkar, A.G. Inhibition of pathogenic bacterial biofilms on PDMS based implants by L. acidophilus derived biosurfactant. BMC Microbiol. 2019, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Tahmourespour, A.; Salehi, R.; Kermanshahi, R.K. Lactobacillus acidophilus-derived biosurfactant effect on gtfB and gtfC expression level in Streptococcus mutans biofilm cells. Braz. J. Microbiol. 2011, 42, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordani, B.; Costantini, P.E.; Fedi, S.; Cappelletti, M.; Abruzzo, A.; Parolin, C.; Foschi, C.; Frisco, G.; Calonghi, N.; Cerchiara, T.; et al. Liposomes containing biosurfactants isolated from Lactobacillus gasseri exert antibiofilm activity against methicillin resistant Staphylococcus aureus strains. Eur. J. Pharm. Biopharm. 2019, 139, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Borsanyiova, M.; Patil, A.; Mukherji, R.; Prabhune, A.; Bopegamage, S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol. 2016, 61, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Juma, A.; Lemoine, P.; Simpson, A.B.J.; Murray, J.; O’Hagan, B.M.G.; Naughton, P.J.; Dooley, J.G.; Banat, I.M. Microscopic investigation of the combined use of antibiotics and biosurfactants on methicillin resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.F.; Tehseen, B.; Sarwar, Y.; Hussain, S.Z.; Khan, W.S.; Raza, Z.A.; Bajwa, S.Z.; Kanaras, A.G.; Hussain, I.; Rehman, A. Biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. J. Hazard. Mater. 2019, 364, 441–448. [Google Scholar] [CrossRef]
- Marangon, C.A.; Martins, V.C.A.; Ling, M.H.; Melo, C.C.; Plepis, A.M.G.; Meyer, R.L.; Nitschke, M. Combination of rhamnolipid and chitosan in nanoparticles boosts their antimicrobial efficacy. ACS Appl. Mater. Interfaces 2020, 12, 5488–5499. [Google Scholar] [CrossRef]
- Suling, W.J.; O’Leary, W.M. Effect of surfactants on antibiotic resistance. Antimicrob. Agents. Chemother. 1975, 8, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Van ‘t Veen, A.; Gommers, D.; Mouton, J.W.; Kluytmans, J.A.J.W.; Krijt, E.J.; Lachmann, B. Exogenous pulmonary surfactant as a drug delivering agent: Influence of antibiotics on surfactant activity. Br. J. Pharmacol. 1996, 118, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.A.; Mortin, L.I.; VanPraagh, A.D.G.; Li, T.; Alder, J. Inhibition of Daptomycin by pulmonary surfactant: In vitro modeling and clinical impact. J. Infect. Dis. 2005, 191, 2149–2152. [Google Scholar] [CrossRef] [PubMed]
- Schwameis, R.; Erdogan-Yildirim, Z.; Manafi, M.; Zeitlinger, M.A.; Strommer, S.; Sauermanna, R. Effect of pulmonary surfactant on antimicrobial activity in vitro. Antimicrob. Agents Chemother. 2013, 57, 5151–5154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.C.; Hugie, C.N.; Kile, M.L.; Navab-Daneshmand, T. Association between heavy metals and antibiotic-resistant human pathogens in environmental reservoirs: A review. Front. Environ. Sci. Eng. 2019, 13, 46. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Effect of biosurfactants on Pseudomonas aeruginosa and Staphylococcus aureus biofilms in a BioFlux channel. Appl. Microbiol. Biotechnol. 2016, 100, 5773–5779. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol. Lett. 2016, 363, fnv224. [Google Scholar] [CrossRef] [Green Version]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Caola, I.; Nollo, G.; Rinaldi, M.; Fracchia, L. Inhibition of Candida albicans biofilm by lipopeptide AC7 coated medical-grade silicone in combination with farnesol. AIMS Bioeng. 2018, 5, 192–208. [Google Scholar] [CrossRef]
- Jung, M.; Lee, S.; Kim, H. Recent studies on natural products as anti-HIV agents. Curr. Med. Chem. 2000, 7, 649–661. [Google Scholar] [CrossRef]
- Seydlová, G.; Svobodová, J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. 2008, 3, 123–133. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Wang, Y.; Li, Y.; Wang, X.; Yang, Q. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J. Virol. 2018, 92, e00809–e00818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Lu, Z.; Zhao, H.; Yang, S. Antiviral activity of antimicrobial lipopeptide from Bacillus subtilis fmbj against Pseudorabies Virus, Porcine Parvovirus, Newcastle Disease Virus and Infectious Bursal Disease Virus in vitro. Int. J. Pept. Res. Ther. 2006, 12, 373–377. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Peng, J.; Li, Y.; Yang, Q. Synthetic surfactin analogues have improved anti-PEDV properties. PLoS ONE 2019, 14, e0215227. [Google Scholar] [CrossRef]
- Hoq, M.M.; Suzutani, T.; Toyoda, T.; Horiike, G.; Yoshida, I.; Azuma, M. Role of gamma delta TCR+ lymphocytes in the augmented resistance of trehalose 6,6′-dimycolate-treated mice to influenza virus infection. J. Gen. Virol. 1997, 78, 1597–1603. [Google Scholar] [CrossRef]
- Shah, V.; Doncel, G.F.; Seyoum, T.; Eaton, K.M.; Zalenskaya, I.; Hagver, R.; Azim, A.; Gross, R. Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob. Agents Chemother. 2005, 49, 4093–4100. [Google Scholar] [CrossRef] [Green Version]
- Gross, R.A.; Shah, V. Anti-Herpes Virus Properties of Various Forms of Sophorolipids. Patent WO2007130738 A1, 15 November 2007. [Google Scholar]
- Gross, R.A.; Shah, V.; Doncel, G. Virucidal Properties of Various Forms of Sophorolipids. U.S. Patent US8648055B2, 11 February 2014. [Google Scholar]
- Remichkova, M.; Galabova, D.; Roeva, I.; Karpenko, E.; Shulga, A.; Galabov, A.S. Anti-herpesvirus activities of Pseudomonas sp. S-17 rhamnolipid and its complex with alginate. Z. Naturforsch. C J. Biosci. 2008, 63, 75–81. [Google Scholar] [CrossRef]
- Jin, L.; Black, W.; Sawyer, T. Application of environment-friendly rhamnolipids against transmission of enveloped viruses like SARS-CoV2. Viruses 2021, 13, 322. [Google Scholar] [CrossRef]
- Smith, M.L.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K.S.M. Biosurfactants: A Covid-19 perspective. Front. Microbiol. 2020, 11, 1341. [Google Scholar] [CrossRef]
- Sandeep, L.; Rajasree, S. Biosurfactant: Pharmaceutical perspective. J. Anal. Pharm. Res. 2017, 4, 00105. [Google Scholar] [CrossRef]
- Nakanishi, M.; Inoh, Y.; Kitamoto, D.; Furuno, T. Nano vectors with a biosurfactant for gene transfection and drug delivery. J. Drug Deliv. Sci. Technol. 2009, 19, 165–169. [Google Scholar] [CrossRef]
- Çelik, P.A.; Manga, E.B.; Çabuk, A.; Banat, I.M. Biosurfactants’ potential role in combating COVID-19 and similar future microbial threats. Appl. Sci. 2021, 11, 334. [Google Scholar] [CrossRef]
- Deres, K.; Schild, H.; Wiesmüller, K.H.; Jung, G.; Rammensee, H.G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature 1989, 342, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Ohadi, M.; Shahravan, A.; Dehghannoudeh, N.; Eslaminejad, T.; Banat, I.M.; Dehghannoudeh, G. Potential use of microbial surfactant in microemulsion drug delivery system: A systematic review. Drug Des. Devel. Ther. 2020, 14, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.C.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Mekonnen, A.; Sidamo, T.; Asres, K.; Engidawork, E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J. Ethnopharmacol. 2013, 145, 638–646. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Lancey Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Attinger, C.; Wolcott, R. Clinically addressing biofilm in chronic wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Clinton, A.; Carter, T. Chronic wound biofilms: Pathogenesis and potential therapies. Lab. Med. 2015, 46, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial biofilms and chronic wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Lydon, H.L.; Baccile, N.; Callaghan, B.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob. Agents Chemother. 2017, 61, e02547-16. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Preservative and irritant capacity of biosurfactants from different sources: A comparative study. J. Pharm. Sci. 2019, 108, 2296–2304. [Google Scholar] [CrossRef]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Zouari, R.; Moalla-Rekik, D.; Sahnoun, Z.; Rebai, T.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Evaluation of dermal wound healing and in vitro antioxidant efficiency of Bacillus subtilis SPB1 biosurfactant. Biomed. Pharmacother. 2016, 84, 878–891. [Google Scholar] [CrossRef]
- Ohadi, M.; Forootanfar, H.; Rahimi, H.R.; Jafari, E.; Shakibaie, M.; Eslaminejad, T.; Dehghannoudeh, G. Antioxidant potential and wound healing activity of biosurfactant produced by Acinetobacter junii B6. Curr. Pharm. Biotechnol. 2017, 18, 900–908. [Google Scholar] [CrossRef]
- Sana, S.; Mazumder, A.; Datta, S.; Biswas, D. Towards the development of an effective in vivo wound healing agent from Bacillus sp. derived biosurfactant using Catla catla fish fat. RSC Adv. 2017, 7, 13668–13677. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Liu, G.; Zhao, B.; Pang, B.; Wu, W.; Ai, C.; Zhao, X.; Wang, X.; Jiang, C.; Shao, D.; et al. Novel biomedical functions of surfactin A from Bacillus subtilis in wound healing promotion and scar inhibition. Agric. Food Chem. 2020, 68, 6987–6997. [Google Scholar] [CrossRef]
- Gupta, S.; Raghuwanshi, N.; Varshney, R.; Banat, I.M.; Srivastava, A.K.; Pruthi, P.A.; Pruthi, V. Accelerated in vivo wound healing evaluation of microbial glycolipid containing ointment as a transdermal substitute. Biomed. Pharmacother. 2017, 94, 11861196. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.; Datta, S.; Biswas, D.R.; Auddy, B.; Gupta, M.; Chattopadhyay, H. Excision wound healing activity of a common biosurfactant produced by Pseudomonas sp. Wound Med. 2018, 23, 47–52. [Google Scholar] [CrossRef]
- More, S.V.; Koratkar, S.S.; Kadam, N.; Agawane, S.; Prabhune, A. Formulation and evaluation of wound healing activity of sophorolipid-sericin gel in wistar rats. Pharmacogn. Mag. 2019, 15, 123–127. [Google Scholar]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial biosurfactants in cosmetic and personal skincare pharmaceutical formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer: Key Facts. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 12 April 2017).
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Alfarouk, K.O.; Stock, C.M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef] [Green Version]
- Dy, G.K.; Adjei, A.A. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J. Clin. 2013, 63, 249–279. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.S.; Ngai, S.C.; Goh, B.H.; Chan, K.G.; Lee, L.H.; Chuah, L.H. Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front. Pharmacol. 2017, 8, 761. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Srirama, K.; Kota, R.K.; Mikkili, I.; Kodali, V.P. Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain. J. King Saud Univ. Sci. 2020, 32, 223–227. [Google Scholar] [CrossRef]
- Klappe, K.; Hummel, I.; Hoekstra, D.; Kok, J.W. Lipid dependence of ABC transporter localization and function. Chem. Phys. Lipids 2009, 161, 57–64. [Google Scholar] [CrossRef]
- Liu, J.; Ma, H.; Wei, T.; Liang, X.J. CO2 gas induced drug release from pH-sensitive liposome to circumvent doxorubicin resistant cells. Chem. Commun. 2012, 48, 4869–4871. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Yin, M.; Hu, X.; Zhuang, X.; Sun, Y.; Guo, Y.; Tan, S.; Zhang, Z. A safe, simple and efficient doxorubicin prodrug hybrid micelle for overcoming tumor multidrug resistance and targeting delivery. J. Control. Release 2016, 235, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef]
- Huang, W.; Lang, Y.; Hakeem, A.; Lei, Y.; Gan, L.; Yang, X. Surfactin-based nanoparticles loaded with doxorubicin to over-come multidrug resistance in cancers. Int. J. Nanomed. 2018, 13, 1723–1736. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Ahmad Khan, M.S.; Singh Cameotra, S.; Safar Al-Thubiani, A. Biosurfactants: Potential applications as immunomodulator drugs. Immunol. Lett. 2020, 223, 71–77. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Park, S.C. Immunomodulatory role of microbial surfactants, with special emphasis on fish. Int. J. Mol. Sci. 2020, 21, 7004. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Kochina, O.A.; Gein, S.V.; Ivshina, I.B.; Chereshnev, V.A. Mechanisms of immunomodulatory and membranotropic activity of trehalolipid biosurfactants (a Review). Appl. Biochem. Microbiol. 2020, 56, 245–255. [Google Scholar] [CrossRef]
- Jensen, P.Ø.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L.; Moser, C.; Williams, P.; Pressler, T.; Givskov, M.; et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 2007, 153, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gennip, M.; Christensen, L.D.; Alhede, M.; Phipps, R.; Jensen, P.Ø.; Christophersen, L.; Pamp, S.J.; Moser, C.; Mikkelsen, P.J.; Koh, A.Y.; et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS 2009, 117, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Dössel, J.; Meyer-Hoffert, U.; Schröder, J.M.; Gerstel, U. Pseudomonas aeruginosa-derived rhamnolipids subvert the host innate immune response through manipulation of the human beta-defensin-2 expression. Cell Microbiol. 2012, 14, 1364–1375. [Google Scholar] [CrossRef]
- Bluth, M.H.; Kandil, E.; Mueller, C.M.; Shah, V.; Lin, Y.Y.; Zhang Dresner, L.; Lempert, L.; Nowakowski, M.; Gross, R.; Schulze, R. Sophorolipids block lethal effects of septic shock in rats in a cecal ligation and puncture model of experimental sepsis. Crit. Care Med. 2006, 34, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bluth, M.H.; Kandil, E.; Mueller, C.M.; Shah, V.; Lin, Y.Y.; Zhang, H.; Dresner, L.; Lempert, L.; Nowakowski, M.; Gross, R.; et al. Sophorolipids decrease IgE production in U266 cells by downregulation of BSAP (Pax5), TLR-2, STAT3 and IL-6. Allergy Clin. Immunol. Pract. 2007, 119, S263. [Google Scholar]
- Kim, K.; Jung, S.Y.; Lee, D.K.; Jung, J.K.; Park, J.K.; Kim, D.K.; Lee, C.H. Suppression of inflammatory responses by surfactin, a selective inhibitor of platelet cytosolic phospholipase A2. Biochem. Pharmacol. 1998, 55, 975–985. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y. Surfactin inhibits immunostimulatory function of macrophages through blocking NK-kappaB, MAPK and Akt pathway. Int. Immunopharmacol. 2009, 9, 886–893. [Google Scholar] [CrossRef]
- Tang, J.S.; Zhao, F.; Gao, H.; Dai, Y.; Yao, Z.H.; Hong, K.; Li, J.; Ye, W.C.; Yao, X.S. Characterization and online detection of surfactin isomers based on HPLC-MS analyses and their inhibitory effects on the overproduction of nitric oxide and the release of TNF-α and IL-6 in LPS-induced macrophages. Mar. Drugs 2010, 8, 2605–2618. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, Y.H.; Kim, E.K.; Ryu, E.Y.; Lee, S.J. Heme oxygenase-1 signals are involved in preferential inhibition of pro-inflammatory cytokine release by surfactin in cells activated with Porphyromonas gingivalis lipopolysaccharide. Chem. Biol. Interact. 2010, 188, 437–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation 2015, 38, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Muehlradt, P.; Barkhausen, T.; Tschernig, T. Pharmaceutical Compositions for Treating Dysregulated Inflammatory Diseases. U.S. Patent US2013079274A1, 28 March 2013. [Google Scholar]
- Layre, E.; Collmann, A.; Bastian, M.; Mariotti, S.; Czaplicki, J.; Prandi, J.; Mori, L.; Stenger, S.; De Libero, G.; Puzo, G.; et al. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem. Biol. 2009, 16, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.H.; Yu, A.L.; Wu, T.N.; Lin, K.-H. Human iNKT Cell Activation Using Glycolipids. World Patent WO2016040369, 17 September 2016. [Google Scholar]
- Guzman, C.A.; Muhlradt, P. Use of a Lipopeptide or Lipoprotein as an Adjuvant in Therapeutic or Prophylactic Vaccinations. World Patent WO2003084568, 16 October 2003. [Google Scholar]
- Moldes, A.; Vecino, X.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J.M. Biosurfactants: The use of biomolecules in cosmetics and detergents. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gomes Rodrigues, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 8; pp. 163–218. [Google Scholar]
- Markets and Markets Biosurfactants Market Analysis Recent Market Developments Industry Forecast to 2016–2022. Available online: https://www.marketsandmarkets.com/Market-Reports/biosurfactant-market-163644922.html (accessed on 11 March 2021).




| Biosurfactant | Function | Application Field |
|---|---|---|
| Mupirocin | Antibacterial | Biomedical and pharmaceutical |
| Oxazolidinone linezolid | Antibacterial | Biomedical and pharmaceutical |
| Daptomycin | Antibacterial | Biomedical and pharmaceutical |
| Caspofungin | Antifungal | Biomedical and pharmaceutical |
| Amphotericin B | Antifungal | Biomedical and pharmaceutical |
| Micafungin | Antifungal | Biomedical and pharmaceutical |
| Anidulafungin | Antifungal | Biomedical and pharmaceutical |
| Rhamnolipids | Emollient, emulsifier | Cosmetic and Personal Skincare |
| Rapeseed sophorolipids | Antimicrobial, cleansing, deodorant, surfactant | Cosmetic and Personal Skincare |
| Hydrolyzed palm sophorolipids | Skin conditioning, skin protecting, surfactant | Cosmetic and Personal Skincare |
| Madhuca longifolia sophorolipids | Antioxidant, antiseborrheic, cleansing, emulsifier, surfactant | Cosmetic and Personal Skincare |
| Sodium surfactin | Cleansing, emulsifying, gel forming, surfactant | Cosmetic and Personal Skincare |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceresa, C.; Fracchia, L.; Fedeli, E.; Porta, C.; Banat, I.M. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics 2021, 13, 466. https://doi.org/10.3390/pharmaceutics13040466
Ceresa C, Fracchia L, Fedeli E, Porta C, Banat IM. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics. 2021; 13(4):466. https://doi.org/10.3390/pharmaceutics13040466
Chicago/Turabian StyleCeresa, Chiara, Letizia Fracchia, Emanuele Fedeli, Chiara Porta, and Ibrahim M. Banat. 2021. "Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants" Pharmaceutics 13, no. 4: 466. https://doi.org/10.3390/pharmaceutics13040466