Synergistic Effect of Growth Factor Releasing Polymeric Nanoparticles and Ultrasound Stimulation on Osteogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of G-PLGA Nanoparticles
2.3. LC/MS Analysis for Quantification of Growth Factors in PLGA Nanoparticles
2.4. Physicochemical Characterization of Particles
2.4.1. Measurements of Particle Properties by Dynamic Light Scattering (DLS) and Scanning Electron Microscopy (SEM)
2.4.2. Characterization of Particle Wetting Properties by a Microscopy
2.4.3. Measurements of Particle Properties by Dynamic Scanning Calorimetry (DSC) and X-ray Diffraction (XRD)
2.5. Low-Intensity Ultrasound Setup
2.6. Release Study of Growth Factors from G-PLGA Particles
2.7. Cell Culture
2.8. Examination of Osteogenic Differentiation from Mesenchymal Stem Cells (MSCs)
2.8.1. Application of Different Cell Culture System for Differentiation
2.8.2. Immunofluorescence Study
2.8.3. Image Analysis
2.9. Data Analysis
3. Results and Discussions
3.1. Physicochemial Characteristics of PLGA Particle Formulations
3.2. DSC and XRD Analyses of PLGA Particles
3.3. Growth Factor Release Profiles of PLGA-Based Formulations
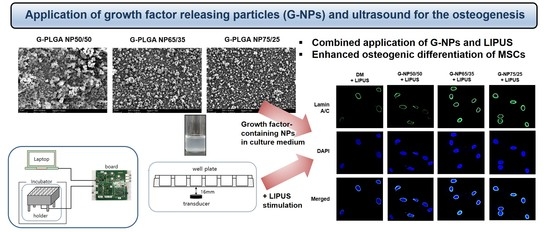
3.4. Application of G-NPs and Ultrasound for The Osteogenic Differentiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Matinlinna, J.P.; Tsoi, J.K.H.; Liu, W.; Cui, X.; Lu, W.W.; Pan, H. Recent developments in biomaterials for long-bone segmental defect reconstruction: A narrative overview. J. Orthop. Transl. 2020, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.A.; Hematti, P.; Kao, W.J. Cell encapsulating biomaterial regulates mesenchymal stromal/stem cell differentiation and macrophage immunophenotype. Stem Cells Translat. Med. 2012, 1, 740–749. [Google Scholar] [CrossRef]
- Wang, X.; Chu, W.; Zhuang, Y.; Shi, D.; Tao, H.; Jin, C.; Dai, K.; Zhao, J.; Gan, Y. Bone mesenchymal stem cell-enriched β-Tricalcium phosphate scaffold processed by the Screen-Enrich-Combine circulating system promotes regeneration of diaphyseal bone non-union. Cell Transplant. 2019, 28, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Fernandez-Moure, J.S.; Fan, D.; Murphy, M.B.; Yazdi, I.K.; Liu, X.; Weiner, B.K.; Tasciotti, E. Biocompatible PLGA-mesoporous silicon microspheres for the controlled release of BMP-2 for bone augmentation. Pharmaceutics 2020, 12, 118. [Google Scholar] [CrossRef] [Green Version]
- Augello, A.; Kurth, T.B.; De Bari, C. Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur. Cell Mater. 2010, 20, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Mazur, M.M.; Buck, A.C.; Wandtke, M.E.; Liu, J.; Ebraheim, N.A. Prospective review of mesenchymal stem cells differentiation into osteoblasts. Orthop. Surg. 2017, 9, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, M.E.; Locatelli, F.; Fibbe, W.E. Mesenchymal stromal cells: A novel treatment modality for tissue repair. Ann. N. Y. Acad. Sci. 2009, 1176, 101–117. [Google Scholar] [CrossRef]
- Yilgor, P.; Hasirci, N.; Hasirci, V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. A 2010, 93, 528–536. [Google Scholar] [CrossRef]
- Amjadian, S.; Seyedjafari, E.; Zeynali, B.; Shabani, I. The synergistic effect of nano-hydroxyapatite and dexamethasone in the fibrous delivery system of gelatin and poly (l-lactide) on the osteogenesis of mesenchymal stem cells. Int. J. Pharm. 2016, 507, 1–11. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Wang, Y.; Varshney, R.R.; Ren, L.; Gong, Y.; Wang, D.-A. Microsphere-based drug releasing scaffolds for inducing osteogenesis of human mesenchymal stem cells in vitro. Eur. J. Pharm. Sci. 2010, 39, 59–67. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Brown, S.E.; Wang, Z.; Krebsbach, P.H. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009, 18, 955–968. [Google Scholar] [CrossRef]
- Facchin, F.; Bianconi, E.; Canaider, S.; Basoli, V.; Biava, P.M.; Ventura, C. Tissue regeneration without stem cell transplantation: Self-healing potential from ancestral chemistry and physical energies. Stem Cells Int. 2018, 2018, 7412035. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering stem cell organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.L.; Dalrymple, G.V. Biological effects of diagnostic ultrasound: A review. Radiology 1978, 126, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Ito, M.; Harada, Y.; Takagi, H.; Ohta, T.; Jingushi, S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J. Bone Miner. Res. 2001, 16, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Samuels, J.A.; Weingarten, M.S.; Margolis, D.J.; Zubkov, L.; Sunny, Y.; Bawiec, C.R.; Conover, D.; Lewin, P.A. Low-frequency (<100 kHz), low-intensity (<100 mW/cm2) ultrasound to treat venous ulcers: A human study and in vitro experiments. J. Acoust. Soc. Am. 2013, 134, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.H.; Park, K.; Park, S.R.; Min, B.-H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: An in vivo study. Tissue Eng. 2006, 12, 75–82. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, T.; Alhadlaq, A.; Wong, B.; Kucharski, C. Ultrasound effect on neural differentiation of gingival stem/progenitor cells. Ann. Biomed. Eng. 2014, 42, 1406–1412. [Google Scholar] [CrossRef]
- Feczkó, T.; Tóth, J.; Dósa, G.; Gyenis, J. Optimization of protein encapsulation in PLGA nanoparticles. Chem. Eng. Proc. 2011, 50, 757–765. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Patel, S.K.; Palmer, K.E.; Devlin, B.; Rohan, L.C. Design of poly (lactic-co-glycolic acid)(PLGA) nanoparticles for vaginal co-delivery of griffithsin and dapivirine and their synergistic effect for HIV prophylaxis. Pharmaceutics 2019, 11, 184. [Google Scholar] [CrossRef] [Green Version]
- Curran, J.M.; Fawcett, S.; Hamilton, L.; Rhodes, N.P.; Rahman, C.V.; Alexander, M.; Shakesheff, K.; Hunt, J.A. The osteogenic response of mesenchymal stem cells to an injectable PLGA bone regeneration system. Biomaterials 2013, 34, 9352–9364. [Google Scholar] [CrossRef]
- García-Díaz, M.; Foged, C.; Nielsen, H.M. Improved insulin loading in poly (lactic-co-glycolic) acid (PLGA) nanoparticles upon self-assembly with lipids. Int. J. Pharm. 2015, 482, 84–91. [Google Scholar] [CrossRef]
- Akter, R.; Rivas, D.; Geneau, G.; Drissi, H.; Duque, G. Effect of lamin A/C knockdown on osteoblast differentiation and function. J. Bone Miner. Res. 2009, 24, 283–293. [Google Scholar] [CrossRef]
- Alcorta-Sevillano, N.; Macías, I.; Rodríguez, C.I.; Infante, A. Crucial role of Lamin A/C in the migration and differentiation of MSCs in bone. Cells 2020, 9, 1330. [Google Scholar] [CrossRef]
- Kim, D.; Maharjan, P.; Jin, M.; Park, T.; Maharjan, A.; Amatya, R.; Yang, J.; Min, K.A.; Shin, M.C. Potential albumin-based antioxidant nanoformulations for ocular protection against oxidative stress. Pharmaceutics 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lwin, W.W.; Puyathorn, N.; Senarat, S.; Mahadlek, J.; Phaechamud, T. Emerging role of polyethylene glycol on doxycycline hyclate-incorporated Eudragit RS in situ forming gel for periodontitis treatment. J. Pharm. Investig. 2020, 50, 81–94. [Google Scholar] [CrossRef]
- Shamarekh, K.S.; Gad, H.A.; Soliman, M.E.; Sammour, O.A. Towards the production of monodisperse gelatin nanoparticles by modified one step desolvation technique. J. Pharm. Investig. 2020, 50, 189–200. [Google Scholar] [CrossRef]
- Maharjan, P.; Jin, M.; Kim, D.; Yang, J.; Maharjan, A.; Shin, M.C.; Cho, K.H.; Kim, M.S.; Min, K.A. Evaluation of epithelial transport and oxidative stress protection of nanoengineered curcumin derivative-cyclodextrin formulation for ocular delivery. Arch. Pharm. Res. 2019, 42, 909–925. [Google Scholar] [CrossRef]
- Lam, K.H.; Hsu, H.S.; Li, Y.; Lee, C.; Lin, A.; Zhou, Q.; Kim, E.S.; Shung, K.K. Ultrahigh frequency lensless ultrasonic transducers for acoustic tweezers application. Biotechnol. Bioeng. 2013, 110, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.W.; Lee, N.S.; Koo, K.M.; Moon, S.; Goo, K.; Jung, H.; Yoon, C.; Lim, H.G.; Shung, K.K. Investigation of ultrasound-mediated intracellular Ca2+ oscillations in HIT-T15 pancreatic β-cell Line. Cells 2020, 9, 1129. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kiio, T.M.; Park, S. Physical properties of nanoparticles do matter. J. Pharm. Investig. 2021, 51, 35–51. [Google Scholar] [CrossRef]
- Nikolakakis, I.; Partheniadis, I. Self-emulsifying granules and pellets: Composition and formation mechanisms for instant or controlled release. Pharmaceutics 2017, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Yonemochi, E.; Hoshino, T.; Yoshihashi, Y.; Terada, K. Evaluation of the physical stability and local crystallization of amorphous terfenadine using XRD–DSC and micro-TA. Thermochim. Acta 2005, 432, 70–75. [Google Scholar] [CrossRef]
- Min, K.A.; Shin, M.C.; Yu, F.; Yang, M.; David, A.E.; Yang, V.C.; Rosania, G.R. Pulsed magnetic field improves the transport of iron oxide nanoparticles through cell barriers. ACS Nano 2013, 7, 2161–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korjamo, T.; Heikkinen, A.T.; Waltari, P.; Mönkkönen, J. The asymmetry of the unstirred water layer in permeability experiments. Pharm. Res. 2008, 25, 1714–1722. [Google Scholar] [CrossRef]
- Bermeo, S.; Vidal, C.; Zhou, H.; Duque, G. Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/β-catenin pathway. J. Cell. Biochem. 2015, 116, 2344–2353. [Google Scholar] [CrossRef]
- Hsu, S.-h.; Kuo, C.-C.; Whu, S.W.; Lin, C.-H.; Tsai, C.-L. The effect of ultrasound stimulation versus bioreactors on neocartilage formation in tissue engineering scaffolds seeded with human chondrocytes in vitro. Biomol. Eng. 2006, 23, 259–264. [Google Scholar] [CrossRef]
- Aliabouzar, M.; Lee, S.j.; Zhou, X.; Zhang, G.L.; Sarkar, K. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells. Biotechnol. Bioeng. 2018, 115, 495–506. [Google Scholar] [CrossRef]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.S.; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyson, M. Non-thermal cellular effects of ultrasound. Br. J. Cancer Suppl. 1982, 5, 165–171. [Google Scholar]
- Katiyar, A.; Duncan, R.L.; Sarkar, K. Ultrasound stimulation increases proliferation of MC3T3-E1 preosteoblast-like cells. J. Ther. Ultrasound 2014, 2, 1. [Google Scholar] [CrossRef]
- Grodzinsky, A.J.; Levenston, M.E.; Jin, M.; Frank, E.H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2000, 2, 691–713. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E.; Frankel, V.; Ruter, A. Ultrasound--an alternative healing method for nonunions? Arch. Orthop. Trauma Surg. 2000, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.D.; Overby, D.R.; Mannix, R.; Ingber, D.E. Cellular adaptation to mechanical stress: Role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J. Cell Sci. 2006, 119, 508–518. [Google Scholar] [CrossRef] [Green Version]








| Formulation | G-NP50/50 | G-NP65/35 | G-NP75/25 | |
|---|---|---|---|---|
| Particle size (nm) | ||||
| Solution in water | Day 1 | 498.4 (±15.2) | 532.3 (±10.5) | 562.7 (±12.4) |
| Day 30 | 485.2 (±10.4) | 510.2 (±4.4) | 520.0 (±8.3) | |
| Spray-dried powder | Day 1 | 502.1 (±8.4) | 542.4 (±7.2) | 534.6 (±9.5) |
| Day 30 | 495.0 (±11.2) | 504.2 (±6.4) | 515.5 (±10.8) | |
| Zeta potential (mV) | ||||
| Solution in water | Day 1 | −8.2 (±2.5) | −5.2 (±3.2) | −8.2 (±3.2) |
| Day 30 | −9.7 (±1.8) | −7.1 (±2.8) | −7.5 (±2.1) | |
| Spray-dried powder | Day 1 | −7.5 (±3.5) | −6.3 (±1.4) | −8.6 (±2.7) |
| Day 30 | −8.5 (±2.4) | −8.2 (±2.2) | −9.5 (±1.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Kim, B.S.; Seo, S.H.; Kim, M.; Kang, Y.G.; Shin, J.-W.; Cho, K.H.; Shin, M.C.; Yoon, C.; Min, K.A. Synergistic Effect of Growth Factor Releasing Polymeric Nanoparticles and Ultrasound Stimulation on Osteogenic Differentiation. Pharmaceutics 2021, 13, 457. https://doi.org/10.3390/pharmaceutics13040457
Jin M, Kim BS, Seo SH, Kim M, Kang YG, Shin J-W, Cho KH, Shin MC, Yoon C, Min KA. Synergistic Effect of Growth Factor Releasing Polymeric Nanoparticles and Ultrasound Stimulation on Osteogenic Differentiation. Pharmaceutics. 2021; 13(4):457. https://doi.org/10.3390/pharmaceutics13040457
Chicago/Turabian StyleJin, Minki, Bo Seok Kim, Sung Ho Seo, Minjeong Kim, Yun Gyeong Kang, Jung-Woog Shin, Kwan Hyung Cho, Meong Cheol Shin, Changhan Yoon, and Kyoung Ah Min. 2021. "Synergistic Effect of Growth Factor Releasing Polymeric Nanoparticles and Ultrasound Stimulation on Osteogenic Differentiation" Pharmaceutics 13, no. 4: 457. https://doi.org/10.3390/pharmaceutics13040457