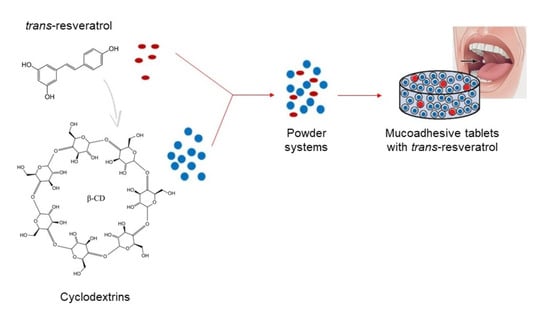
Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preformulation Studies
2.2.1. Preparation of the Cyclodextrin Systems
- –
- Method 1 (dry mixing, DM)—RSV and CD starting material powders in a molar ratio of 1:1 and 1:2 were added to an agate mortar and pestle. The materials were subjected to a dry mechanochemical activation for 60 min.
- –
- Method 2 (kneading with an ethanol/water mixture, Kn)—RSV and CD in a molar ratio of 1:1 and 1:2 were added to an agate mortar and pestle and kneaded with an ethanol-water (1:3 v/v) mixture until the solvent evaporated.
- –
- Method 3 (solvent evaporation, Evap)—The aqueous solution of CD was added to an ethanol solution of RSV (in an RSV/CD molar ratio of 1:1 and 1:2). The mixture was evaporated using Rotavapor® R-300 (Buchi) at 45 °C until dry.
2.2.2. Identity Study of Solid Samples
Powder X-ray Diffraction Characterization (PXRD)
Fourier Transform Infrared Spectroscopy (FT-IR)
High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) Method Development and Validation
2.2.3. Evaluation of Pharmaceutical Properties of Solid Samples
Dissolution Studies
Permeability Studies
Antioxidant Activity—DPPH and CUPRAC Assays
2.3. Formulation Studies
2.3.1. Tableting Process
2.3.2. Tablet Characterization
2.3.3. In Vitro Release Studies
2.3.4. Mucoadhesive Properties
In Vitro Assessment of Mucin-Biopolymer Bioadhesive Bond Strength
Determination of the Ex Vivo Mucoadhesive Properties (Maximum Detachment Force and Work of Mucoadhesion)
Determination of the Residence Time
3. Results and Discussion
3.1. Preformulation Studies
3.2. Formulation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Popova, C.; Dosseva-Panova, V.; Panov, V. Microbiology of Periodontal Diseases. A Review. Biotechnol. Biotechnol. Equip. 2013, 27, 3754–3759. [Google Scholar] [CrossRef]
- Eid Abdelmagyd, H.A.; Ram Shetty, D.S.; Musa Musleh Al-Ahmari, D.M. Herbal medicine as adjunct in periodontal therapies—A review of clinical trials in past decade. J. Oral. Biol. Craniofac. Res. 2019, 9, 212–217. [Google Scholar] [CrossRef]
- Kala, R.; Tollefsbol, O.T.; Li, Y. Potential of Resveratrol in Inhibiting Cancer and Slowing Aging. J. Nutr. Food Sci. 2012, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, S.; Khazaei, M.; Kazemi, S.; Yaghini, J. Resveratrol as a supplemental treatment for periodontitis. Dent. Res. J. 2012, 9, 655–657. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Peng, W.; Zhu, Y. Effects of resveratrol on cariogenic virulence properties of Streptococcus mutans. BMC Microbiol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Andrade, E.F.; Orlando, D.R.; Araújo, A.M.S.; De Andrade, J.N.B.M.; Azzi, D.V.; De Lima, R.R.; Lobo-Júnior, A.R.; Pereira, L.J. Can Resveratrol Treatment Control the Progression of Induced Periodontal Disease? A Systematic Review and Meta-Analysis of Preclinical Studies. Nutrients 2019, 11, 953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonechi, C.; Lamponi, S.; Donati, A.; Tamasi, G.; Consumi, M.; Leone, G.; Rossi, C.; Magnani, A. Effect of resveratrol on platelet aggregation by fibrinogen protection. Biophys. Chem. 2017, 222, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Serrero, G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J. Cell. Physiol. 1999, 179, 297–304. [Google Scholar] [CrossRef]
- Mgbonyebi, O.P.; Russo, J.; Russo, I.H. Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int. J. Oncol. 1998, 12, 865–869. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Oyenihi, O.R.; Oyenihi, A.B.; Adeyanju, A.A.; Oguntibeju, O.O. Antidiabetic Effects of Resveratrol: The Way Forward in Its Clinical Utility. J. Diabetes Res. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific Structural Determinants Are Responsible for the Antioxidant Activity and the Cell Cycle Effects of Resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [Green Version]
- Spogli, R.; Bastianini, M.; Ragonese, F.; Iannitti, R.G.; Monarca, L.; Bastioli, F.; Nakashidze, I.; Brecchia, G.; Menchetti, L.; Codini, M.; et al. Solid Dispersion of Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Microparticles Improves Oral Bioavailability. Nutrients 2018, 10, 1925. [Google Scholar] [CrossRef] [Green Version]
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability is the Problem, What is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.; Oatis, J., Jr.; Walle, K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-Da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as Functional Excipients: Methods to Enhance Complexation Efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J.M.S. Cyclodextrins as excipients in tablet formulations. Drug Discov. Today 2018, 23, 1274–1284. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J.M.S. Hydroxypropyl-β-Cyclodextrin and β-Cyclodextrin as Tablet Fillers for Direct Compression. AAPS PharmSciTech 2018, 19, 2710–2718. [Google Scholar] [CrossRef]
- De Vries, K.; Strydom, M.; Steenkamp, V. Bioavailability of resveratrol: Possibilities for enhancement. J. Herb. Med. 2018, 11, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral mucosal drug delivery: Clinical pharmacokinetics and therapeutic applications. Clin. Pharmacokinet. 2002, 41, 661–680. [Google Scholar] [CrossRef]
- Blanchard, O.L.; Friesenhahn, G.; Javors, M.A.; Smoliga, J.M. Development of a Lozenge for Oral Transmucosal Delivery of Trans-Resveratrol in Humans: Proof of Concept. PLoS ONE 2014, 9, e90131. [Google Scholar] [CrossRef]
- Vaz-Da-Silva, M.; Loureiro, A.; Falcao, A.; Nunes, T.; Rocha, J.-F.; Fernandes-Lopes, C.; Soares, E.; Wright, L.; Almeida, L.; Soares-Da-Silva, P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int. J. Clin. Pharmacol. Ther. 2008, 46, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nicolas, J.M.; Garcia-Carmona, F. Aggregation state and pKa values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef]
- Ansari, M.; Sadarani, B.; Majumdar, A. Optimization and evaluation of mucoadhesive buccal films loaded with resveratrol. J. Drug Deliv. Sci. Technol. 2018, 44, 278–288. [Google Scholar] [CrossRef]
- Martins, I.C.F.; Raposo, N.R.B.; Mockdeci, H.R.; Polonini, H.C.; Ferreira, A.D.O.; Fabri, G.M.C.; Chaves, M.D.G.A.M. Delivering Resveratrol on the Buccal Mucosa Using Mucoadhesive Tablets: A Potential Treatment Strategy for Inflammatory Oral Lesions. Curr. Drug Deliv. 2018, 15, 254–259. [Google Scholar] [CrossRef]
- Bojanowski, K.; Bojanowski, R. Two methods of oral delivery of resveratrol: A case study. J. Aging Res. Clin. Pract. 2015, 4, 185–189. [Google Scholar]
- Paczkowska, M.; McDonagh, A.F.; Bialek, K.; Tajber, L.; Cielecka-Piontek, J. Mechanochemical activation with cyclodextrins followed by compaction as an effective approach to improving dissolution of rutin. Int. J. Pharm. 2020, 581, 119294. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of curves with an emphasis on in vitro dissolution profiles. Pharm. Tech. 1996, 20, 64–74. [Google Scholar]
- Chen, K. The PAMPA Work Flow and Comparison of UV-Plate Reader Method vs. LC/MS Method. Available online: https://vdocuments.net/the-pampa-work-flow-and-comparison-of-uv-plate-permeability-formula-has-taken.html (accessed on 4 March 2021).
- Yee, S. In Vitro Permeability Across Caco-2 Cells (Colonic) Can Predict In Vivo (Small Intestinal) Absorption in Man—Fact or Myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of Pentacyclic Triterpenoids-Rich Callus Extract of Chaenomeles japonica (Thunb.) Lindl. ex Spach on Viability, Morphology, and Proliferation of Normal Human Skin Fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. ACTA 2007, 160, 413–419. [Google Scholar] [CrossRef]
- Uniformity of dosage units. In European Pharmacopoeia, 10th ed.; Council of Europe Portal: Strasbourg, France, 2020; Chapter 2.9.40; pp. 398–400.
- Tye, C.K.; Sun, C.; Amidon, G.E. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Hassan, E.E.; Gallo, J.M. A Simple Rheological Method for the in Vitro Assessment of Mucin-Polymer Bioadhesive Bond Strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Nakamura, F.; Ohta, R.; Machida, Y.; Nagai, T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int. J. Pharm. 1996, 134, 173–181. [Google Scholar] [CrossRef]
- Huang, X.; Dai, Y.; Cai, J.; Zhong, N.; Xiao, H.; McClements, D.J.; Hu, K. Resveratrol encapsulation in core-shell biopolymer nanoparticles: Impact on antioxidant and anticancer activities. Food Hydrocoll. 2017, 64, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Wang, F.; Guo, Z.; Zhao, Y.L. Preparation and characterization of resveratrol/hydroxypropyl-beta-cyclodextrin inclusion complex using supercritical antisolvent technology. J. Food Process. Eng. 2012, 35, 1–10. [Google Scholar] [CrossRef]
- Kumpugdee-Vollrath, M.; Ibold, Y.; Sriamornsak, P. Solid state characterization of trans resveratrol complexes with different cyclodextrins. JAASP 2012, 1, 125–136. [Google Scholar]
- Silva, A.F.R.; Monteiro, M.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Inclusion Complex of Resveratrol with γ-Cyclodextrin as a Functional Ingredient for Lemon Juices. Foods 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of Resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertacche, V.; Lorenzi, N.; Nava, D.; Pini, E.; Sinico, C. Host–Guest Interaction Study of Resveratrol with Natural and Modified Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 279–287. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro charac-terisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech. 2011, 12, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.M. Transport of Small Molecules. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Aree, T.; Jongrungruangchok, S. Structure–antioxidant activity relationship of β-cyclodextrin inclusion complexes with olive tyrosol, hydroxytyrosol and oleuropein: Deep insights from X-ray analysis, DFT calculation and DPPH assay. Carbohydr. Polym. 2018, 199, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Folch-Cano, C.; Jullian, C.; Speisky, H.; Olea-Azar, C. Antioxidant activity of inclusion complexes of tea catechins with β-cyclodextrins by ORAC assays. Food Res. Int. 2010, 43, 2039–2044. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants. 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and Antioxidants. Crit. Rev. Food Sci. Nutr. 2013, 54, 251–276. [Google Scholar] [CrossRef] [PubMed]
- EMA. Cyclodextrins used as excipients. Eur. Med. Agency 2017, 23, 1–16. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 19 March 2021).
- Sun, C.; Grant, D.J.W. Influence of Crystal Structure on the Tableting Properties of Sulfamerazine Polymorphs. Pharm. Res. 2001, 18, 274–280. [Google Scholar] [CrossRef]
- Salústio, P.J.; Pontes, P.; Conduto, C.; Sanches, I.; Carvalho, C.; Arrais, J.; Marques, H.M.C. Advanced Technologies for Oral Controlled Release: Cyclodextrins for Oral Controlled Release. AAPS PharmSciTech 2011, 12, 1276–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Iqbal, M.; Akhtar, N.; Khan, H.M.S.; Ullah, A.; Uddin, M.; Khan, M.T. Assessment of xanthan gum based sustained release matrix tablets containing highly water-soluble propranolol HCl. ACTA Pol. Pharm. Drug Res. 2013, 70, 283–289. [Google Scholar]
- Gavini, E.; Rassu, G.; Haukvik, T.; Lanni, C.; Racchi, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of cyclodextrins. J. Drug Target. 2009, 17, 168–179. [Google Scholar] [CrossRef]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Improved viscoelasticity of xanthan gum through self-association with surfactant: β-cyclodextrin inclusion complexes for applications in enhanced oil recovery. Polym. Eng. Sci. 2014. [Google Scholar] [CrossRef]









| F1 | F2 | F3 | F4 | F5 | |
|---|---|---|---|---|---|
| Complexation method | DM | DM | DM | DM | Evap |
| Content (mg) of compounds in one tablet | |||||
| RSV | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| α-CD | 85.0 | - | - | - | - |
| β-CD | - | 99.0 | - | - | - |
| γ-CD | - | - | 128.0 | - | - |
| HP-β-CD | - | - | - | 114.0 | 114.0 |
| xanthan gum | 10.5 | 11.9 | 14.8 | 13.4 | 13.4 |
| magnesium stearate | 1.2 | 1.3 | 1.6 | 1.5 | 1.5 |
| sum | 116.7 | 132.2 | 164.4 | 148.9 | 148.9 |
| Compression Pressure | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| 150 MPa | >180 | >180 | 120 | 10 | >180 |
| 200 MPa | >180 | >180 | 100 | 10 | >180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnicka, K.; Ruchała, M.A.; Cielecka-Piontek, J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics 2021, 13, 417. https://doi.org/10.3390/pharmaceutics13030417
Paczkowska-Walendowska M, Dvořák J, Rosiak N, Tykarska E, Szymańska E, Winnicka K, Ruchała MA, Cielecka-Piontek J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics. 2021; 13(3):417. https://doi.org/10.3390/pharmaceutics13030417
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Jakub Dvořák, Natalia Rosiak, Ewa Tykarska, Emilia Szymańska, Katarzyna Winnicka, Marek A. Ruchała, and Judyta Cielecka-Piontek. 2021. "Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment" Pharmaceutics 13, no. 3: 417. https://doi.org/10.3390/pharmaceutics13030417