Excipients in the Paediatric Population: A Review
Abstract
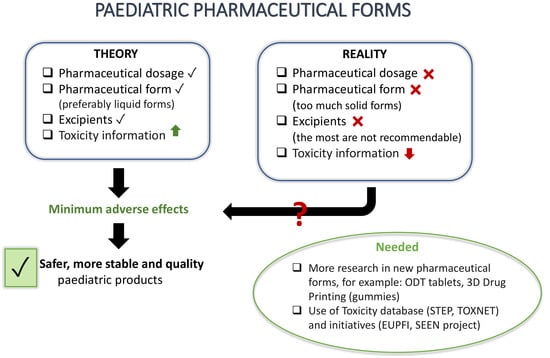
:1. Introduction
- Table 1. Toxicity database.
- Table A1. Most important characteristics of the excipients discussed in this review (in alphabetical order).
- Table A2. Examples of solid and semi-solid medicines used in Spain for the paediatric population: List of excipients and relevant characteristics of the pharmaceutical form (PF) (performed consultation of CIMA database, September 2020).
- Table A3. Examples of liquid medicines used in paediatrics: List of excipients and relevant characteristics of (PF).
- Table A4. Examples of FDA-registered drugs used in paediatrics (FDA database and DAILYMED October 2020).
- Table A5. Examples of liquid formulations for paediatric use in research articles.
2. Paediatric Regulatory Context
- Ensure that these medicines were of good quality.
- Verify that paediatric medicines were produced following ethical and legitimate research, that children were not subjected to unnecessary trials.
- Improve the accessibility and availability of information on drug use in the paediatric population.
- Such regulations led to the establishment of the Paediatric Committee (PDCO), whose main function was to regulate the studies that companies should conduct in children as part of a Paediatric Research Plan (PRP) [6].
- The Paediatric Regulation consists of [7]:
- Regulation (EC) 1901/2006 of the European Parliament and of the Council on medicinal products for paediatric use; and
- Regulation (EC) 1902/2006, an amending regulation in which changes were made to the original text in relation to the European Commission’s decision-making procedures.
- Excipient function in formulation and possible alternatives.
- Safety profile of the excipient for children in target age groups, based on a unique and daily exposure.
- Expected duration of treatment: short term (a single dose for a few days) or long term (weeks and/or months).
- Severity of the condition to be treated and therapeutic alternatives.
- Patient acceptability, including palatability.
- Allergies and sensitization. Children suffer from sensitization problems more commonly than adults. Applicants should avoid, when possible, excipients with known potential to cause sensitization or allergies.
Examples of Databases and Initiatives for the Registration of Information on Excipients Used in the Paediatric Population
- Identify the problems and challenges associated with the development of paediatric formulation and consider ways to obtain better medicines and dosage forms clinically relevant to children.
- Promote early pharmaceutical consideration for the development of paediatric medicines.
- Identify potential information and knowledge gaps in the development of paediatric formulations.
- Improve the availability of information from paediatric formulations.
- Excipients known to be toxic/have general safety issues.
- Frequency of appearance as contaminants or toxics in paediatrics (where applicable).
- Evidence in the toxicity literature in paediatrics. The above criteria were applied to identify excipients for inclusion in the STEP database. Excipients were shortlisted/prioritized through surveys within EU and US PFI members.
- Propylene glycol (PG)
- Ethanol
- Polysorbate 80
- Benzyl alcohol
- Parabens (propyl, methyl, ethyl and butyl)
- Benzalkonium chloride
- Aspartame
- Sorbitol
- Benzoic acid
- Sodium benzoate
- Validate the STEP Version 1 database against the potential needs of end users to ensure that the STEP database meets users’ expectations.
- Evaluate the functionality and usability of data application by
- Ensuring proper ease of use (navigation), understanding and user satisfaction.
- Characterizing how easy it is to perform a task using the database.
- Identifying problems in interaction with systems.
- Evaluate the impact of this database on the development of paediatric medicines.
- Establish viable recommendations to further improve the functionality of the system and increase its beneficial effects on the development of paediatric medicines.
3. Excipients: Functions and Main Adverse Effects
3.1. Diluents
3.1.1. Lactose
3.1.2. Starch
3.1.3. Microcrystalline Cellulose
3.2. Solvents
3.2.1. Water
3.2.2. Ethyl Alcohol (Ethanol)
3.2.3. Propylene Glycol (PG)
- Hyperosmolar syndrome in burnt children with topical arsenic sulfadiazine ointment containing PG.
- Precipitation of irreversible deafness in pretermits who received a multivitamin complex containing PG.
- Parenterally it is possible to observe haemolysis, seizures, respiratory depression, hypertension.
- Contact dermatitis is topically observed.
3.2.4. Glycerol
3.2.5. Polyethylene Glycol (PEG)
3.3. Coating Agents
Phthalates
3.4. Preservatives
3.4.1. Sodium Benzoate
3.4.2. Benzyl Alcohol
3.4.3. Benzalkonium Chloride
3.4.4. Thiomersal
3.4.5. Parabens
3.5. Antioxidants
3.5.1. Sulphites
3.5.2. Propyl Gallate
3.6. Sweeteners
3.6.1. Sucrose
3.6.2. Sorbitol
3.6.3. Mannitol
3.6.4. Aspartame
3.6.5. Saccharine
3.6.6. Sucralose
3.7. Surfactants
Polysorbates
3.8. Colorants
3.9. Excipients not Recommended in Paediatrics and Paediatric Formulations
- Approximately 100% of the formulations shown here carry at least one excipient not recommended for the paediatric population.
- Benzalkonium chloride, methyl para hydroxybenzoate and propyl para hydroxybenzoate are some of the most commonly used preservatives in solid and semi-solid formulations for paediatric use, even though they are considered to be potentially toxic in neonates.
- Sucrose, aspartame and mannitol are used as sweetener. 100% of the oral solid formulations collected in Table A4 carry at least one excipient of these: 40% of formulations carry mannitol and aspartame; 20% carry the 3 excipients; 20% sucrose and aspartame and the remaining 20% only sucrose.
- Propylene glycol is another excipient commonly used in solid formulations as a solvent, moisturizer and preservative. Caution should be exercised in children under 4 years of age and neonates, as propylene glycols, at high doses, may cause alterations in the Central Nervous System, in addition to other side effects discussed in the previous sections of this paper.
- Microcrystalline cellulose, methylcellulose and ethyl cellulose are one of the most commonly used excipients in solid formulations. They have no major side effects, but in high amounts they can cause a laxative effect.
- Most of the solid formulations collected in Table A2 use flavourings such as grape essence, lemon flavouring, caramel cream aroma or orange essence, in order to achieve a better palatability. The main drawback of their incorporation into paediatric formulations is that they usually have a complex and poorly known composition [49].
- Lanolin is an excipient used in pastes and ointments, which are frequently used in the paediatric population. This excipient may cause skin hypersensitivity reactions, which is why caution should be exercised in patients with known sensitivity issues [50].
- Ethanol, sorbitol and propylene glycol, despite being contraindicated in paediatrics, especially ethanol, are still included in some paediatric formulations.
- The addition of non-recommended sweeteners, such as sucrose, sucralose or sodium saccharine, is also seen in these paediatric formulations.
- The addition of preservatives in paediatric formulations should be avoided as much as possible, and if necessary, in the least amount. Parabens are among the safest preservatives in paediatrics, yet others that are not recommended are still used (e.g., Table A3: sodium benzoate, benzoic acid and benzyl alcohol). Benzalkonium chloride, despite not being recommended for asthmatic patients, is used for the formulation of most eye drops, nasal drops and gothic drops.
- Like the other examples, there is also frequent use of sweeteners (fructose, sucrose, sucralose, aspartame and sodium saccharine).
- Benzalkonium chloride is one of the most commonly used preservatives in ophthalmic and nasal drops, as shown in Table A4. It is usually a safe excipient, but can cause serious adverse effects, such as bronchoconstriction in asthmatic patients, ototoxicity in erotic preparations or respiratory failure in infants who ingest this excipient, this adverse effect being the most severe.
4. Promising Pharmaceutical Form in the Paediatric Population: ODT and 3D Drug Printing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Purposes of the STEP Database
- Serve as a public base for evidence regarding the safety and toxicity of excipients in order to allow the pharmaceutical industry, academics, pharmacists, physicians and regulators to make informed decisions.
- Improve prospects of identifying potential security issues in the early stages of the development process when excipients are selected.
- Help highlight any relationships between exposure and evidence of clinically significant toxicity in the paediatric age group in general, or in paediatric subpopulations.
- Identify possible differences in expression, types or patterns of toxicity in children compared to adults. Provide a basis for assessing the need to generate new data for paediatric medicines (e.g., bridge studies, juvenile toxicity studies, etc.), in order to clarify what kind of new data, knowledge gaps or studies may be needed.
- Support companies with their regulatory presentations with easily available information.
- Support and improve research activities by providing a platform to share unreleased data and available data with corporate entities.
Appendix B
| Excipient | Functions | DAI * | Recommendations | Adverse Effects | References |
|---|---|---|---|---|---|
| Aspartame | Artificial Sweetener | 40 mg/kg |
|
| [29,35] |
| Benzalkonium chloride | Preservative | NA |
|
| [29] |
| Benzyl alcohol | Preservative | 5 mg/kg |
| In new-borns and children under 3 years of age cause:
| [29,35,36,39,40,41,42,43,44] |
| Ethyl alcohol | Solvent and preservative | 6 mg/kg/dose (<6 years) | Paediatric formulations should not exceed the following limits of ethanol:
|
| [17,26,27,29,31,32] |
| Glycerol | Solvent, sweetener, viscosizer and preservative | 10 g/dose |
|
| [1,29] |
| Lactose | Diluent | NA |
| Symptoms of lactose intolerance: severe abdominal pain, flatulence, bloating or swelling and diarrhoea.
| [1,28] |
| Parabens | Preservative | 10 mg/kg |
|
| [29,35,36] |
| Phthalates | Coating agents (plasticizers) | NA |
|
| [28] |
| Polyethylene glycol | Solvent, suspensor and viscosity agent | 10 mg/kg |
|
| [1,28] |
| Polysorbates | Dispersing, emulgent, surfactants, solubilizing and moisturizing agents | NA |
|
| [25,42] |
| Propyl gallate | Antioxidant | NA |
|
| [29] |
| Propylene glycol | Solvent, moisturizing and preservative |
|
|
| [29,33,34] |
| Saccharine | Sweetener | 2.5 mg/kg |
|
| [29,48] |
| Sorbitol | Sweetener and diluent |
|
|
| [28,29,30] |
| Starch | Diluent and added | NA |
|
| [29] |
| Sucralose | Sweetener | 15 mg/kg |
| Alters the composition of the digestive tract microbiome
| [29] |
| Sucrose | Sweetener | NA |
|
| [29] |
| Sulphites | Antioxidant | NA |
|
| [29] |
| Tartrazine, quinolines, triphenylmethane, xanthines | Colorants | NA |
|
| [28,29] |
| Thiomersal | Preservative | NA |
|
| [28,29] |
Appendix C
| Pharmaceutical Form | Excipients | API | Pharmaceutical Form Characteristics | References | ||
|---|---|---|---|---|---|---|
| SOLID PREPARATIONS | POWDERS | Example 1: Amoxicillin Normon 250 mg/5 mL EFG Oral Suspension Powder | Saccharose, Glucose, Methyl parahydroxybenzoate (E-218), Propyl parahydroxybenzoate (E-216), Anhydrous sodium citrate, Colloidal silica and Orange essence | Amoxicillin |
| [29,57,58] |
| Example 2: Azithromycin Sandoz 200 mg/5 mL EFG Oral Suspension Powder | Sucrose, Xanthan gum (E415), Hydroxypropyl cellulose, Anhydrous trisodium phosphate, Colloidal anhydrous silica (E551), Aspartame (E951), Aroma of caramel cream and Titanium dioxide (E171) | Azithromycin | ||||
| GRANULATED | Example 1: Paediatric Gelocatil 325 mg Granules | Calcium carbonate, Sodium hydrogen carbonate, Citric acid anhydrous, Anhydrous sodium citrate, Aspartame (E-951), Sucrose, Mannitol (E-421), Amorphous silica, Glycerol die-stearate type 1, Croscarmellose sodium, Sodium glycolate starch type A (potato starch) gluten-free, Ethyl cellulose, Hydroxypropyl methylcellulose and Polyethylene glycol 400 | Paracetamol |
| [29,59] | |
| SOLID PREPARATIONS | ORAL DISPERSIBLE TABLETS (ODT) | Example 1: Apiretal 325 mg oral dispersible tablets | Ethyl cellulose, Microcrystalline cellulose, Crospovidone, Aspartame (E-951), Colloidal silica, Mannitol, Talco, Magnesium stearate and Grape essence | Paracetamol | As advantages of oral dispersible tablets, the following stand out:
| [54,60,61] |
| Example 2: Junifen 200 mg lemon-flavored oral dispersible tablets | Ethyl cellulose, Precipitated silicon dioxide, Hypromellose, Mannitol, Aspartame (E-951), Croscarmellose sodium, Magnesium stearate and Lemon flavouring | Ibuprofen | ||||
| SOLID PREPARATIONS | SUPPOSITORIES | Example 1: Febectal Infants 150 mg Suppositories | Colloidal anhydrous silica, Solid semi-synthetic glycerides | Paracetamol | As advantages, the following stand out:
| [29,62] |
| SEMI-SOLID PREPARATIONS | GELS | Example 1: Fenistil 1 mg/g Gel | Benzalkonium chloride, Disodium edetate, Carbomer, Sodium hydroxide, Propylene glycol amd Purified water | Dimethindene maleate |
| [29,63] |
| SEMI-SOLID PREPARATIONS | CREAMS | Example 1: Perme-Cure 5% Cream | Butylhydroxytoluene (E-321), Castor Oil, Deionized water, Steareth-2, Ceteareth-2-Phosphate, Sosa to the 20 %, Vitamin E acetate, Phenonip, Citric acid, Disodium edetate and Scent | Permethrin cis:trans (25:75) | As advantages, the following stand out:
| [64] |
| OINTMENTS | Example 1: Oftacilox 3 mg/g Ophthalmic Ointment | Liquid paraffin and White Vaseline | Ciprofloxacin |
| [29,65] | |
| PASTES | Example 1: Anti-congestive Cusi (Paste Lassar) | Lanolin (wool fat), Liquid Vaseline and Stringy Vaseline |
|
| [29,66] | |
| SEMI-SOLID PREPARATIONS | NON-CREAM EMULSIONS | Example 1: Lactisona 10 mg/mL Skin Emulsion | Carbomer 940, 1,3-dimethylol-5,5-dimethyl hydantoin, Dihydro-acetic acid, Pyrrolidone sodium carboxylate, Lactic acid, Sodium hydroxide, Stearyl alcohol, Glycerol stearate, Cetyl alcohol, Isopropyl palmitate, Mineral oil, Myristyl lactate, Fragrance and Water | Hydrocortisone |
| [50,67] |
Appendix D
| Pharmaceutical Form | Excipients | API | Pharmaceutical Form Characteristics | References | ||
|---|---|---|---|---|---|---|
| LIQUID PREPARATIONS | ORAL SOLUTIONS | Example 1: Diazepam 2 mg/5 mL Solution without sugar | Sodium Docusate, Aluminium silicate and Magnesium, Propylene glycol, Raspberry Flavour, Sodium Saccharine, Precool Erythrosine (E127), Sorbic Acid (E200), Propyl para hydroxybenzoate, Methyl para hydroxybenzoate, Sorbitol, Liquid (Non-Crystalized) (E420) and Glycerol (E422) | Diazepam | As advantages, the following stand out:
| [29,49,68,69,70] |
| Example 2: Paracetamol Level 100 mg/mL Oral Solution | Citric acid, Sodium hydroxide, Sucrose, Propylene glycol, Macrogol, Strawberry Essence, Cochineal Red A (Ponceau 4R) (E-124), Hydrochloric Acid 5 N and Purified Water | Paracetamol | ||||
| Example 3: Diazepam Intensol™ Oral Solution 5 mg/mL * Do not use in children under 6 months of age | Alcohol, Yellow D&C 10, Polyethylene glycol, Succinic Acid and Water | Diazepam |
| [71,72] | ||
| Example 4: Prednisolone 10 mg/mL Oral Solution | Sodium Methyl para hydroxybenzoate, Sodium Propyl para hydroxybenzoate, Glycerol, Sodium Saccharine, Sodium Edetate, Sodium Aqueous solutions of medicinal substances that areDihydrate, Orange flavour (contains propylene glycol), Sodium hydroxide and Purified Water | Prednisolone | ||||
| LIQUID PREPARATIONS | ORAL SOLUTIONS | Example 5: Ozalin | Citric acid monohydrate, Gamma-cyclodextrin, Sucralose, Orange flavour (contains 70–80% ethanol), Sodium hydroxide, injectable water | Midazolam | See “Pharmaceutical Form Characteristics (Oral Solutions)” section of the previous page | [73,74,75] |
| Example 6: Flumil 20 mg/mL Oral Solution | Para-Hydroxybenzoate Methyl (E218), Sodium Benzoate (E211), Sodium Edetate, Carmellose Sodium, Sodium saccharine, Sodium Cyclamate, Sucralose, Raspberry Aroma, Sodium Hydroxide and Purified Water | Acetyl cysteine | ||||
| Example 7: Paediatric Lanacordin 0.05 mg/mL * Including newborns and premature | Sucrose, Ethanol, Tartrazine (E-102), Anhydrous Sodium Phosphate, Citric Acid (E-330), Methyl Hydroxybenzoate, Lime Essential Oil, Propylene glycol (E-1520) and Purified Water | Digoxin | ||||
| LIQUID PREPARATIONS | ORAL SUSPENSIONS | Example 1: Paracetamol 120 mg/5 mL Oral Suspension | Propylene glycol, Methyl Hydroxybenzoate, Propyl Hydroxybenzoate, Xanthan Gum, 70% Sorbitol Solution, Sucrose, Mango flavour and Purified Water | Paracetamol | As advantages, the following stand out:
| [76,77] |
| Example 2: Junior Parapaed 120 mg/5 mL Oral Suspension | Ethanol, Polysorbate 80, Glycerol, Magnesium and Aluminium silicate, Liquid maltitol syrup, Sodium saccharine (E954), xanthan gum, cherry flavour, sodium benzoate, Citric acid monohydrate and purified water | Paracetamol | ||||
| LIQUID PREPARATIONS | ORAL SUSPENSIONS | Example 3: Mycostatin 100.000 UI/mL Oral Suspension | Sucrose, 96% ethanol, Carmellose sodium, Cinnamic aldehyde, Mint Essence, Cherry Aroma, Anhydrous Disodium Hydrogen phosphate, Glycerol (E-422), Methyl para hydroxybenzoate, Propyl para hydroxybenzoate, Sodium Hydroxide, Hydrochloric Acid and Purified Water | Nystatin | Disadvantages include:
| [78,79] |
| Example 4: Paediatric Algidrin 20 mg/mL Oral Suspension * Do not give to children under 3 months of age | Microcrystalline cellulose, Carboxymethylcellulose sodium, Sorbitol (E-420), Maltitol (E-965), Beta-cyclodextrin, Sodium Saccharine, Sucralose (E-955), Forest Fruit Aroma, Allura AC Red Colouring (E-129), Methyl para hydroxybenzoate, Ethyl para hydroxybenzoate, Propyl para hydroxybenzoate and Purified Water | Ibuprofen (Lysine) | ||||
| LIQUID PREPARA-TIONS | ORAL SUSPEN-SIONS | Example 5: Paediatric Septrin 8 mg/40 mg/mL Oral Suspension * Suitable for infants from 6 weeks of age | Sorbitol, Glycerol (E-422), Dispersible Cellulose, Carmellose Sodium, Polysorbate 80, Methyl para hydroxybenzoate, Sodium Benzoate, Sodium Saccharine, Banana flavour (Propylene Glycol E-1520, Sodium Citrates E-331), Ethanol 96°, Vanilla flavour (Benzyl Alcohol, Caramel Colour E-150d, Propylene Glycol E-1520, Glycerol E-422, Water), Purified Water. |
| See “Pharmaceutical Form Characteristics (Oral Suspension)” section of the previous page | [80] |
| LIQUID PREPARATIONS | ELIXIRS | Example 1: Paracetamol Elixir Pediátrico 120 mg/5 mL | Ethanol 96° (10% v/v), Propylene glycol, Inverted Syrup, Amaranth Solution (E123), Glycerol, Glycerine, Chloroform and Concentrated Raspberry Juice | Paracetamol |
| [29,81,82] |
| Example 2: Lanoxin Elixir * Fit for premature neonates | Methyl Hydroxybenzoate, Sucrose, Sodium Phosphate Anhydrous, Citric Acid Monohydrate, Quinine Yellow, Ethanol (96%), Propylene Glycol, Lime flavour and Purified Water | Digoxin | ||||
| SYRUPS | Example 1: Daleron Syrup 120 mg/5 mL | Sorbitol, Glycerol, Xanthan Gum, Maltitol, Microcrystalline Cellulose, Croscarmellose Sodium, Sodium Benzoate, Citric Acid, Pineapple flavour, Riboflavin and Purified Water | Paracetamol |
| [29,83,84] | |
| Example 2: Loratadine 5 mg/mL Syrup Oral Solution | Propylene glycol, Glycerol, Sodium Benzoate, Citric Acid Monohydrate, Sucrose, Peach flavour and Purified Water | Loratadine | ||||
| LIQUID PREPARA-TIONS | SYRUPS | Example 3: Polaramine 0.4 mg/mL Syrup * Not suitable for children under 2 years old | Ethanol, Sucrose, Sodium Citrate, Sodium Chloride, Sorbitol, Methyl paraben, Propyl paraben, Menthol, Apricot flavour, Orange flavour, Ponceau 4R Colouring (E-124) and Purified Water | Dexchlorpheni-ramine maleate | See “Pharmaceutical Form Characteristics (Syrups)” section of the previous page | [85,86] |
| Example 4: Paediatric Mucosan 3 mg/mL Syrup | Hydroxyethyl cellulose, Sucralose, Benzoic Acid (E-210), Wild Berry Aroma, Vanilla Aroma and Purified Water | Ambroxol hydrochloride | ||||
| LIQUID PREPARATIONS | ORAL DROPS IN SOLUTION | Example 1: Romillary 15 mg/mL Oral drops in Solution * Not recommended for use in children under 2 years of age | Propylene glycol, anhydrous ethanol, Flavourings: coriander oil, orange essential oil and lemon tetraroma, macrogol glycerol ricinolate (chromophore EL), Methyl para hydroxybenzoate, Propyl para hydroxybenzoate, sodium saccharine, citric acid monohydrate, sodium hydroxide and purified water | Hydrobromide dextromethorphan |
| [87,88,89,90] |
| Example 2: Alerlisin 10 mg/mL Oral Drops in Solution * Do not use in children under 2 years of age | Glycerol, Propylene glycol (E-1520), Sodium Saccharine, Methyl para hydroxybenzoate, Propyl para hydroxybenzoate, Sodium Acetate, Glacial Acetic Acid and Purified Water | Cetirizine hydrochloride | ||||
| LIQUIDPREPARA-TIONS | ORAL DROPS IN SOLUTION | Example 3: Paediatric Cleboril 62.5 g Oral Drops in Solution | Benzoic acid (E-210), Sodium hydroxide and purified water | Clebopride malate | See “Pharmaceutical Form Characteristics (Oral Drops in Solution)” section of the previous page | [91,92,93] |
| Example 4: Fluor Lacer 1.4 mg/mL Oral Drops * Indicated for tooth decay prophylaxis in children 1–6 years old | Sodium Saccharine, Propylene glycol, Methyl para hydroxybenzoate, Propyl para hydroxybenzoate, Disodium edetate, Cochineal Red Colouring (E-124), Strawberry Aroma and Purified Water | Sodium Fluoride | ||||
| Example 5: Hydropolivit Oral Drops in Solution * Recommended for children over 2 years old | Propylene glycol, Polysorbate 80, Sorbitol 70% (E-420), Glycerol (E-422), Sodium Saccharine, Sodium Edetate, Monothioglycerol, Methyl para hydroxybenzoate, Butylhydroxyanisole (E-320), Banana Essence, Vanilla Essence, Sodium Hydroxide and Purified Water | -Retinol palmitate Cholecalciferol Alpha-tocopherol acetate Riboflavin Pyridoxine hydrochloride Ascorbic acid Biotin Nicotinamide | ||||
| LIQUID PREPARATIONS | ORAL DROPS INSUSPENSION | Example 1: Zamene 22.75 mg/mL Oral Drops in Suspension * Special interest in paediatrics. Not recommended in children under 2 months of age. | Aluminium and Magnesium silicate, Carboxymethylcellulose sodium, Benzyl alcohol, 70% Sorbitol, Polysorbate 80, Acetic Acid and Purified Water | Deflazacort | They have the same characteristics as oral drops in solution | [94,95] |
| Example 2: Dezacor 22.75 mg/mL Oral Drops in Suspension * Special interest in paediatrics. Not recommended in children under 2 months of age. | Sorbitol solution 70%, Carboxymethylcellulose sodium, Aluminium silicate and magnesium, Polysorbate 80, Benzyl Alcohol, Sucralose, Tropical Fruit Aroma, Citric Acid Monohydrate, Sodium Hydroxide and Purified Water | Deflazacort | ||||
| OPHTHALMIC DROPS OR COLLYRIUMS | Example 1: Atropine BP 1.0% (w/v)/Vistatropin 1.0% (w/v) Eye drops in solution | Benzalkonium chloride in solution and purified water | Atropine sulphate |
| [29,68,96,97] | |
| Example 2: Chibroxin 3 mg/mL Collyrium in solution | Sodium Acetate, Benzalkonium Chloride, Disodium Edetate, Concentrated Hydrochloric Acid, Sodium Chloride and Water for Injections | Norfloxacin | ||||
| LIQUID PREPARATIONS | NASAL DROPS | Example 1: Rhinovin® Children’s 0.5 mg/mL Nasal Drops in Solution * Do not use in children under 6 years of age | Dihydrogen phosphate of sodium dihydrate, disodium phosphate dodecahydrate, disodium Edetate, Benzalkonium Chloride, Sorbitol (E420), Hypromellose, Sodium Chloride and Purified Water | Xylometazoline hydrochloride |
| [29,68,98,99] |
| Example 2: Utabon Children 0.25 mg/mL Nasal Drops in Solution * Do not use in children under 6 years of age | Benzalkonium chloride, anhydrous disodium hydrogen phosphate, Sodium dihydrogen phosphate dihydrate, glycine (E-640), Sorbitol (E-420) and Purified water | Oxymetazoline hydrochloride | ||||
| OTIC DROPS | Example 1: Otic cetraxal 3 mg/mL Otic drops en Solución * Indicated in adults and child | Lactic acid, Povidone, Anhydrous Glucose, Propylene glycol, Methyl para hydroxybenzoate, Propyl para hydroxybenzoate, Hydrochloric Acid and Purified Water | Ciprofloxacin |
| [29,100,101] | |
| Example 2: Otix Otic Drops in Solution * Do not administer in children under 2 years of age | Benzalkonium Chloride, Sulphuric acid, Sodium Chloride, Sodium Hydroxide, Tribasic Sodium Citrate, Polysorbate 80, Citric Acid and Purified Water |
| ||||
| LIQUID PREPARATIONS | OTIC DROPS | Example 3: Ciproxin Simple 3 mg/mL Otic Drops in Solution * Not recommended for children under 1 year old | Benzalkonium Chloride, Sodium Acetate Trihydrate, Glacial Acetic Acid, Mannitol (E-421), Disodium Edetate, Hydrochloric Acid and/or Sodium Hydroxide and Purified Water | Ciprofloxacin hydrochloride | See “Pharmaceutical Form Characteristics (Otic Drops)” section of the previous page | [102] |
| PARENTERAL PREPARATIONS FOR INJECTION | INTRAVENOUS | Example 1: Digoxin Kern Pharma 0.25 mg/mL solution for injection * including premature neonates | Ethanol, Propylene Glycol, Citric Acid Anhydrous, Bi-sodium Anhydrous Phosphate and Bi-distillate Water. | Digoxin |
| [29,103] |
Appendix E
| Pharmaceutical Form | Excipients | Active Principle | Age | References | ||
|---|---|---|---|---|---|---|
| LIQUID PREPARATIONS | ORAL SOLUTIONS | Abilify Solution Oral | Disodium edetate, fructose (200 mg per mL), glycerine, dl-lactic acid, methylparaben, propylene glycol, propylparaben, sodium hydroxide, sucrose (400 mg per mL), and purified water. The Oral solution is flavoured with natural orange cream and other natural flavours | Aripiprazole | 6 to 18 years | [104] |
| Demerol Solution Oral | Benzoic acid, flavour, liquid glucose, purified water, saccharin sodium | Meperidine hydrochloride | Adult and paediatric patients | [105] | ||
| Diazepam Oral Solution (Lannett Company) | Polyethylene glycol, propylene glycol, non-crystallizing sorbitol solution, sodium citrate anhydrous, bitterness modifier flavour, anhydrous citric acid, peppermint flavour, mint flavour, FD&C Network No. 40 aluminium lake, D&C Yellow No. 10 aluminium lake and purified water | Diazepam (5 mg/5 mL) | Children from 6 months | [106] | ||
| ORAL SUSPENSIONS | Adzenys ER (Extend release) | Purified water, sorbitol, propylene glycol, xanthan gum, natural orange flavour, methacrylic acid and methyl methacrylate copolymer, sodium polystyrene sulfonate, vegetable oil, triethyl citrate, methylparaben, citric acid, sucralose, propylparaben, orange colour (FD&C Yellow No. 6), and polyethylene glycol | Amphetamine | 6 to 17 years | [107] | |
| Children’s Tylenol® Cold + Cough + Sore Throat Oral Suspension | Anhydrous citric acid, D&C network No. 33, FD&C network No. 40, flavours, glycerine, microcrystalline cellulose and sodium carboxymethyl cellulose, purified water, sodium benzoate, sorbitol solution, sucralose, xanthan gum | Acetaminophen 160 mg Dextromethorphan hydrobromide 5 mg | 4 to 11 years | [108] | ||
| ORAL SUSPENSIONS | Dyanavel XR (Extend release) | Anhydrous citric acid, bubble-gum flavour, glycerine, methylparaben, modified food starch, polysorbate 80, povidone, polyvinyl acetate, propylparaben, sodium lauryl sulphate, sodium polystyrene sulfonate, sucralose, triacetin and xanthan gum | Amphetamine | Children from 6 years | [109] | |
| SYRUPS | Midazolam hydrochloride syrup | Anhydrous Citric Acid, D&C Network No. 33, edetate disodium, glycerine, sodium benzoate, sorbitol, Water, Hydrochloric Acid, Sodium Citrate | Midazolam hydrochloride | Children from 6 months | [110] | |
| LIQUID PREPARATIONS | OTIC DROPS | Ciprofloxacin and dexamethasone suspension/drops | Benzalkonium chloride, boric acid, edetate disodium, acetic acid, sodium acetate, sodium chloride, sodium hydroxide, tyloxapol, water, hydrochloric acid, hydroxyethyl cellulose (3000 cps at 1%) |
| Children from 6 months | [111] |
| OPHTHALMIC DROPS OR COLLYRIUMS | ALLERGY EYE DROPS- ketotifen fumarate solution/ drops | Benzalkonium chloride 0.01%, glycerine, purified water. may contain hydrochloric acid and/or sodium hydroxide (to adjust PH). | Ketotifen (0.025 %) (equivalent to ketotifen fumarate 0.035 %) | Children from 3 years. Children under 3 years of age: consult to doctor | [112] | |
| NASAL DROPS | LITTLE REMEDIES DECONGESTANT NASAL DROPS phenylephrine hydrochloride liquid | Benzalkonium chloride, glycerine, polyethylene glycol, potassium phosphate monobasic, purified water, Sodium EDTA, sodium phosphate dibasic | Phenylephrine hydrochloride 1.25 mg/mL | Children | [113] | |
| ORAL DROPS | BIO-G-TUSS PAEDIATRIC DROPS (solution) | Citric acid, grape flavour, glycerine, methylparaben, polyethylene glycol, propylparaben, purified water, Sodium citrate, sucralose |
| Children | [114] | |
| SOLI PREPARATIONS | CHEWABLE TABLET | Children’s Motrin—Ibuprofen Tablet, Chewable | Acesulfame potassium, ammonium glycyrrhizin, aspartame, carnauba wax, croscarmellose sodium, hypromellose, magnesium stearate, mannitol, natural and artificial flavours, silicon dioxide, sodium lauryl sulphate, soybean oil, succinic acid | Ibuprofen 100 mg | 2 to 11 years | [115] |
| Acetaminophen Children’s | Citric acid, crospovidone, D&C network No. 27 aluminium lake, D&C network No. 30 aluminium lake, dextrates hydrated, ethyl cellulose, flavours, magnesium stearate, mannitol, polyethylene, stearic acid, sucralose | Acetaminophen 80 mg | 2 to 6 years | [116] | ||
| TABLETS | Diazepam Tablet | Anhydrous lactose, magnesium stearate, cellulose microcrystalline, FD&C blue n. 1 | Diazepam 10 mg | Children from 6 months | [117] | |
| Dexamethasone 1.5 mg tablet | Lactose monohydrate, magnesium stearate, maltodextrin, corn starch, sucrose | Dexamethasone 1.5 mg | It depends on the pathology | [118] | ||
Appendix F
| Formula | Pharmaceutical Form | Excipients | Active Principle (Dose) | Age | Stability (Stability in Use) | References |
|---|---|---|---|---|---|---|
| Organic solvent-based formulation of lorazepam (Oral Solution) | Oral solution | PEG 400 (10% v/v), Propylene glycol (3% m/v), Glycerol (87% v/v) and Orange essence (0.1%) | Lorazepam (1 mg/mL) | Children 1 month to 12 years old | 12 months at 4 °C (Stability in use: 4 weeks) | [119] |
| Oral solution of amlodipine besylate for children | Oral solution | Sucrose jarabe (32% m/v), Methylparaben (solution 15% m/v) (0.3% m/v) and Purified water (75%) | Amlodipine Besylate (0.5 mg/mL) | Paediatric Population (children and teenagers) | 12 months at 4 °C (Stability in use: 18 weeks) | [120] |
| Oral tizanidine hydrochloride, Formulation for hospital use | Oral solution | CMC (carboxymethyl cellulose) (0.5%), Potassic sorbate (0.15%), Sucralose (0.10%), Citric acid and Purified water | Tizanidine Hydrochloride (1 g/mL) | Paediatric Population | 70 days at 15–30 °C, 2–8 °C and 40 °C | [121] |
| Paediatric oral formulation of clonidine hydrochloride | Oral solution | Sucrose syrup (20% v/v), Raspberry essence (0.05%), Methyl paraben solution 15% (1% m/v), Citric acid monohydrate (1% m/v), Disodium hydrogen phosphate (1.8% m/v) and Purified water | Clonidine HCL (50 µg/mL) | Paediatric Population | 9 months at room temperature, protected from light | [122] |
| Oral liquid formulation of clonidine hydrochloride for paediatric patients | Oral solution | Potassic sorbate, Sucrose and Monohydrate citric acid | Clonidine hydrochloride (20 µg/mL) | Paediatric Patients (all ages) | 90 days at 5 °C (cooling) (Stability in use: 42 days at 5 °C) | [123] |
| Paediatric oral formulations of sodium dichloroacetate | Oral solution | Vehicle Mascagni (% w/v): Sucralose (0.02%), Hydroxyethyl cellulose (0.2%), Citric acid (0.09%), Sodium citrate (0.09%) and Potassium sorbate (0.18%) | Sodium dichloroacetate (DCA) (9.5% w/v) | Paediatric Patients | 3 months at 4 °C and 25 °C (Stability in use: 1 month to 4 °C) | [124] |
| Furosemide solutions for personalized paediatric administration | Oral solution (extemporaneous) | Solution I: Buffer carbonate-bicarbonate (pH) (10 mL) Excipient for syrup (cps 100 mL) (ACOFARMA): sucrose, water, sorbitol, glycerine, aroma, citric acid, methyl paraben, potassium sorbate, sodium phosphate and colorant. Solution II: Buffer carbonate-bicarbonate (pH) (10 mL) -Excipient for syrup—without sugars (cps 100 mL) (ACOFARMA): sodium saccharine, xanthan gum, water, sorbitol, glycerine, aroma, citric acid, sodium citrate, methyl paraben, propyl paraben, potassium sorbate, sodium phosphate and colorant. | Furosemide (2 mg/mL) | Paediatrics | 60 days at 4 and 25 °C | [125] |
| Formulation comprising acetaminophen, especially for paediatrics (PATENT) | Oral solution (nano-emulsion) | NF glyceryl mono linoleate (5–30%, preferably 8-26% w/v), PEG-35 castor oil (30–60%, preferably 39–46% w/v), NF diethylene glycol mono ethyl ether (20–45%, preferably 24–40% w/v) and Water | Paracetamol (5–18% w/v) | Paediatrics | NA | [126] |
| Paediatric formulations of ursodeoxycholic acid from oral administration | Oral suspension | Glycerol (20%), Methyl cellulose 1000 (1% v/v) and Purified water | Ursodeoxycholic acid (UDCA) (1.5 mg/mL) | Paediatric Population | 30 days at 25 °C or in fridge | [127] |
| Oral paediatric formulation of hydrochlorothiazide | Oral suspension | Glycerol (20%), Methyl cellulose 1000 (1% v/v), Citric acid (pH corrector) and Water | Hydrochlorothiazide (2 mg/mL) | Paediatric Population in general | 3 weeks at 5 °C and protected from light | [128] |
| Oral suspension of clindamycin HCL with ion exchange resin for paediatric use | Oral suspension | Glycerine (30% w/v), Sucralose (3%), Aroma of maple syrup (7%), Grape aroma (10%), Cremophor RH 40 (15%), Xanthan gum (0.2%) and Deionized water (cps 5 mL) | Clindamycin HCL resin (Amberlite IRP 69) (5.5% w/v) | Paediatric Population | 1 month at 25 °C | [129,130] |
| Isoniazid suspension formulated with cationic resin for paediatric use | Oral suspension | Sorbitol solution 70% USP (4.9 mL/ 5 mL), USP monohydrate citric acid (50 mg/5 mL) and USP potassic sorbate (5 mg/5 mL) | Isoniazid resin/Kyron T-134 100 mg/5 mL/200 mg/5 mL | Paediatric Population | 3 months at 40 °C (accelerated stability study) | [131] |
| Paediatric xylometazoline nasal spray formulation | Nasal Spray | Sodium colatum (105 mg/10 mL), PEG 400 (1.35 mL/10 mL), Sodium carboxy methyl cellulose (10 mg/10 mL), Glycerine (0.15 mL/10 mL), Methyl paraben (3.3 mg/10 mL), Sodium chloride and Purified water (cps 10 mL) | Xylometazoline HCl (5 mg/10 mL) | Paediatric Population | 12 months at 25 °C | [132] |
References
- Cañete, C.R.; García, M.P.; García, B.P.; Cabañas, M.J.P. Formulación magistral y excipientes en pediatría. El Farma-céutico. Hospitales 2018, 213, 22–28. [Google Scholar]
- Salunke, S.; Brandys, B.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: Part 2—The pilot version. Int. J. Pharm. 2013, 457, 310–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, G. Safety of Excipients in Pediatric Formulations—A Call for Toxicity Studies in Juvenile Animals? Child 2015, 2, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency (EMA); Committee for Medicinal Products for Human Use (CHMP); Paediatric Committee (PDCO). Guideline on Pharmaceutical Development of Medicines for Paediatric Use. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 1 June 2020).
- Dunne, J. The European Regulation on medicines for paediatric use. Paediatr. Respir. Rev. 2007, 8, 177–183. [Google Scholar] [CrossRef]
- European Medicines Agency. European Network of Paediatric Research at the European Medicines Agency (Enpr-EMA). Available online: https://www.ema.europa.eu/en/partners-networks/networks/european-network-paediatric-research-european-medicines-agency-enpr-ema (accessed on 1 June 2020).
- EUR-Lex. Regulation EC No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. Available online: https://eur-lex.europa.eu/eli/reg/2006/1901/oj (accessed on 1 July 2020).
- European Medicines Agency. 10-Year Report to the European Commission. Available online: https://ec.europa.eu/health/sites/health/files/files/paediatrics/2016_pc_report_2017/ema_10_year_report_for_consultation.pdf (accessed on 2 June 2020).
- European Medicines Agency. ICH E11(R1) Guideline on Clinical Investigation of Medicinal Products in the Pediatric Population. Step 5 [Internet]. Available online: https://www.ema.europa.eu/en/ich-e11r1-step-5-guideline-clinical-investigation-medicinal-products-pediatric-population (accessed on 2 June 2020).
- Turner, M.; Catapano, M.; Hirschfeld, S.; Giaquinto, C. Paediatric drug development: The impact of evolving regulations. Adv. Drug Deliv. Rev. 2014, 73, 2–13. [Google Scholar] [CrossRef] [PubMed]
- WHO Library Cataloguing-in-Publication Data: WHO Model Formulary for Children 2010. Based on the Second Model List of Essential Medicines for Children 2009. Available online: http://www.who.int/bookorders (accessed on 5 June 2020).
- WHO World Health Organization. Model List of Essential Medicines for Children. Available online: https://apps.who.int/iris/bitstream/handle/10665/325772/WHO-MVP-EMP-IAU-2019.07-eng.pdf (accessed on 6 June 2020).
- Daousani, C.; Karalis, V.D. Paediatric Medicines: Regulatory and Scientific Issues. Drug Res. 2017, 67, 377–384. [Google Scholar] [CrossRef] [PubMed]
- FDA, Alyson Karesh MD. Pediatric Drug Development: Regulatory Expectations. Available online: https://www.fda.gov/files/drugs/published/Pediatric-Drug-Development--Regulatory-Expectations.pdf (accessed on 7 June 2020).
- FDA. Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/nonclinical-studies-safety-evaluation-pharmaceutical-excipients (accessed on 7 June 2020).
- Walsh, J.; Mills, S. Conference Report: Formulating Better Medicines for Children: 4th European Paediatric Formulation Initiative Conference. Ther. Deliv. 2013, 4, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, N. Ethanol exposure through medicines commonly used in paediatrics. Arch. Dis. Child. Educ. Pract. 2014, 100, 101–104. [Google Scholar] [CrossRef]
- Tuleu, C.; Breitkreutz, J. Educational Paper: Formulation-related issues in pediatric clinical pharmacology. Eur. J. Nucl. Med. Mol. Imaging 2012, 172, 717–720. [Google Scholar] [CrossRef]
- Salunke, S.; Liu, F.; Batchelor, H.; Walsh, J.; Turner, R.; Ju, T.R.; Tuleu, C.; on behalf of European Paediatric Formulation Initiative (EuPFI). European Paediatric Formulation Initiative (EuPFI)—Formulating Ideas for Better Medicines for Children. AAPS PharmSciTech 2016, 18, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Salunke, S. Development and Evaluation of Database of Safety and Toxicity of Excipients for Paediatrics (STEP Database). Ph.D. Thesis, University College London, London, UK, 2017. Available online: https://discovery.ucl.ac.uk/id/eprint/1546094/ (accessed on 20 July 2020).
- Salunke, S.; Tuleu, C. The STEP database through the end-users eyes—USABILITY STUDY. Int. J. Pharm. 2015, 492, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Salunke, S.; Giacoia, G.; Tuleu, C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database. Part 1—A need assessment study. Int. J. Pharm. 2012, 435, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Fonger, G.C.; Hakkinen, P.; Jordan, S.; Publicker, S. The National Library of Medicine’s (NLM) Hazardous Substances Data Bank (HSDB): Background, recent enhancements and future plans. Toxicology 2014, 325, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeur, K.S.; Hertel, S.A.; Lundstrøm, K.E.; Holst, H. Safe excipient exposure in neonates and small children—Protocol for the SEEN project. Dan Med. J. 2017, 64, A5324. [Google Scholar]
- Fabiano, V.; Mameli, C.; Zuccotti, G.V. Paediatric pharmacology: Remember the excipients. Pharmacol. Res. 2011, 63, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Soremekun, R.; Ogbuefi, I.; Aderemi-Williams, R. Prevalence of ethanol and other potentially harmful excipients in pediatric oral medicines: Survey of community pharmacies in a Nigerian City. BMC Res. Notes 2019, 12, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Buckley, L.A.; Salunke, S.; Thompson, K.; Baer, G.; Fegley, D.; Turner, M.A. Challenges and strategies to facilitate formulation development of pediatric drug products: Safety qualification of excipients. Int. J. Pharm. 2018, 536, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Parneli, C.; Srivari, Y.; Meiki, Y.; Mohammed, A.; Sri, V. Pharmaceutical excipients and paediatric formulations. Chem. Today 2012, 30, 56–61. [Google Scholar]
- Peiré, M.A.G. Farmacología Pediátrica, 1st ed.; Ediciones Journal: Ciudad Autónoma de Buenos Aires, Argentina, 2019. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Zuccotti, G.V.; Fabiano, V. Safety issues with ethanol as an excipient in drugs intended for pediatric use. Expert Opin. Drug Saf. 2011, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA), Committee for Human Medicinal Products (CHMP). Questions and Answers on Ethanol in the Context of the Revision of the Guideline on ‘Excipients in the Label and Package Leaflet of Medical Products for Human Use’ (CPMP/463/00). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-ethanol-context-revision-guideline-excipients-label-package-leaflet-medicinal_en.pdf (accessed on 10 March 2020).
- Allegaert, K. Propylene Glycol in Neonates: Never Prescribed, Frequently Administered, Hardly Evaluated. J. Clin. Toxicol. 2012, 2, 113. [Google Scholar] [CrossRef]
- Allegaert, K.; Vanhaesebrouck, S.; Kulo, A.; Cosaert, K.; Verbesselt, R.; Debeer, A.; De Hoon, J. Prospective assessment of short-term propylene glycol tolerance in neonates. Arch. Dis. Child. 2010, 95, 1054–1058. [Google Scholar] [CrossRef]
- Hidalgo, M.E.P.; Sánchez, B.N. Formas Farmacéuticas Líquidas Orales (II): Excipientes. Panor. Actual Medicam. 2016, 40, 842–848. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5685059 (accessed on 20 May 2020).
- Nellis, G.; Metsvaht, T.; Varendi, H.; Toompere, K.; Lass, J.; Mesek, I.; Nunn, A.J.; A Turner, M.; Lutsar, I. Potentially harmful excipients in neonatal medicines: A pan-European observational study. Arch. Dis. Child. 2015, 100, 694–699. [Google Scholar] [CrossRef]
- Nellis, G. The Use of Excipients in Medicines Administered to Neonates in Europe. Ph.D. Thesis, University of Tartu, Tartu, Estonia, 2017. Available online: http://rahvatervis.ut.ee/handle/1/6757 (accessed on 3 May 2020).
- Graham, S.; Turner, M. European Study of Neonatal Exposure to Excipients (ESNEE). Infant 2011, 7, 196–199. Available online: http://www.infantjournal.co.uk/pdf/inf_042_ien.pdf (accessed on 3 May 2020).
- Nahata, M.C. Safety of “inert” additives or excipients in paediatric medicines. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F392–F393. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, A.; Currie, A.E.; Turner, M.A.; Field, D.J.; Mulla, H.; Pandya, H.C. Toxic additives in medication for pre-term infants. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, 236–240. Available online: https://fn.bmj.com/content/94/4/F236 (accessed on 20 May 2020). [CrossRef] [Green Version]
- Committee on Drugs "Inactive" Ingredients in Pharmaceutical Products: Update (Subject Review). Pediatry 1997, 99, 268–278. [CrossRef] [PubMed] [Green Version]
- Fister, P.; Urh, S.; Karner, A.; Krzan, M.; Paro-Panjan, D. The prevalence and pattern of pharmaceutical and excipient exposure in a neonatal unit in Slovenia. J. Matern. Neonatal Med. 2015, 28, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). Reflection Paper: Formulations of Choice for the Paediatric Population Agreed by Paediatric Working Party & Quality Working Party Adoption by Chmp for Release for Consultation End of Consultation (Deadline for Comments). Available online: https://www.ema.europa.eu/en/formulations-choice-paediatric-population (accessed on 20 March 2020).
- Valeur, K.S.; Holst, H.; Allegaert, K. Excipients in Neonatal Medicinal Products: Never Prescribed, Commonly Administered. Pharm. Med. 2018, 32, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulí, T.C. Tratado de Farmacia Galenica., 1st ed.; Luzán 5, S.A. de Ediciones: Madrid, Spain, 1993. [Google Scholar]
- Vally, H.; La Misso, N. Adverse reactions to the sulphite additives. Gastroenterol. Hepatol. Bed Bench 2012, 5, 16–23. [Google Scholar]
- Duro, D.; Rising, R.; Cedillo, M.; Lifshitz, F. Association between infantile colic and carbohydrate malabsorption from fruit juices in infancy. Pediatry 2002, 109, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Nakama, K.; Dos Santos, R.; Serpa, P.; Maciel, T.; Haas, S. Organoleptic excipients used in pediatric antibiotics. Arch. Pédiatrie 2019, 26, 431–436. [Google Scholar] [CrossRef]
- Punín, E.C.; Ballester, A.V.; Dávila, C.P.; Varela, J.J.C.; López, M.J.O.; Arias, J.D.; Segado, M.I.J.; Herrero, L.P.; Fortes, S.G.; Piñeiro, G.C.; et al. Aspectos Prácticos de la Farmacotecnia en un Servicio de Farmacia. Situación Actual [Online]; Piñeiro, G.C., Ed.; Master Line & Prodigio S.L.: Madrid, Spain, 2011; Available online: www.sefh.es/bibliotecavirtual/FARMACOTECNIA/AspectosPracticos.pdf (accessed on 13 October 2020).
- Euskadi.eus. Boletín INFAC. Año 2019. Volumen 27. Nº 3- Excipientes: ¿Sustancias Inertes? Available online: https://www.euskadi.eus/boletin-infac-ano-2019-volumen-27/web01-a2cevime/es/ (accessed on 13 October 2020).
- Gryczke, A.; Schminke, S.; Maniruzzaman, M.; Beck, J.; Douroumis, D. Development and evaluation of orally disintegrating tablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surf. B Biointerfaces 2011, 86, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Piplani, M.; Sharma, P.C.; Kaushik, D.; Nanda, S. Orally Disintegrating Tablets—Friendly to Pediatrics and Geriatrics. Arch. Appl. Sci. Res. 2010, 2, 35–48. Available online: www.scholarsresearchlibrary.com/articles/orally-disintegrating-tablets--friendly-to-pediatrics-and-geriatrics.pdf (accessed on 14 November 2020).
- Aguilar-Díaz, J.E.; García-Montoya, E.; Suñe-Negre, J.M.; Pérez-Lozano, P.; Miñarro, M.; Ticó, J.R. Predicting orally disintegrating tablets formulations of ibuprophen tablets: An application of the new SeDeM-ODT expert system. Eur. J. Pharm. Biopharm. 2012, 80, 638–648. [Google Scholar] [CrossRef]
- Arvapalli, S.; Swamy, D.; Shyamala. Oral Disintegration Tablets: An Updated Review. IAJPS 2019, 6, 6926–6934. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, C.; Festag, M.; Kishore, R.S.; Roethlisberger, D.; Schmitt, G. Pediatric Safety of Polysorbates in Drug Formulations. Children 2019, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- AEMPS. CIMA. Ficha Técnica Amoxicilina Normon 250 mg/5 mL Polvo para Suspensión Oral EFG. Available online: https://cima.aemps.es/cima/dochtml/ft/63354/FT_63354.html (accessed on 1 July 2020).
- AEMPS. CIMA. Ficha Técnica Azitromicina Sandoz 200 mg/5 mL Polvo para Suspensión Oral EFG. Available online: https://cima.aemps.es/cima/dochtml/ft/71180/FT_71180.html#6-datos-farmac-uticos (accessed on 1 July 2020).
- AEMPS. CIMA. Ficha Técnica Gelocatil Pediátrico 325 mg Granulado. Available online: https://cima.aemps.es/cima/dochtml/ft/72343/FT_72343.html#6-datos-farmac-uticos (accessed on 1 July 2020).
- AEMPS. CIMA. Ficha Técnica Apiretal 325 mg Comprimidos Bucodispersables. Available online: https://cima.aemps.es/cima/dochtml/ft/70571/FT_70571.html#6-datos-farmac-uticos (accessed on 1 July 2020).
- AEMPS. CIMA. Ficha Técnica Junifen 200 mg Comprimidos Bucodispersables Sabor Limón. Available online: https://cima.aemps.es/cima/dochtml/ft/64966/FT_64966.html#6-datos-farmac-uticos (accessed on 5 July 2020).
- AEMPS. CIMA. Ficha Técnica Febrectal Lactantes 150 mg Supositorios. Available online: https://cima.aemps.es/cima/dochtml/ft/45930/FT_45930.html#6-datos-farmac-uticos (accessed on 5 July 2020).
- AEMPS. CIMA. Ficha Técnica Fenistil 1 mg/g Gel. Available online: https://cima.aemps.es/cima/dochtml/ft/54083/FT_54083.html (accessed on 9 July 2020).
- AEMPS. CIMA. Ficha Técnica Perme-Cure 5 % Crema. Available online: https://cima.aemps.es/cima/pdfs/ft/64052/FT_64052.pdf (accessed on 9 July 2020).
- AEMPS. CIMA. Ficha Técnica Oftacilox 3 mg/g Pomada Oftálmica. Available online: https://cima.aemps.es/cima/dochtml/ft/64904/FT_64904.html#1-nombre-del-medicamento (accessed on 9 July 2020).
- AEMPS. CIMA. Ficha Técnica Anticongestiva Cusí (Pasta Lassar). Available online: https://cima.aemps.es/cima/dochtml/ft/33302/FT_33302.html#6-datos-farmac-uticos (accessed on 9 July 2020).
- AEMPS. CIMA. Ficha Técnica Lactisona 10 mg/mL Emulsión Cutánea. Ficha Técnica. Available online: https://cima.aemps.es/cima/dochtml/ft/59709/FichaTecnica_59709.html (accessed on 9 July 2020).
- Eichholtz, F. Tratado de Farmacología, 1st ed.; Editorial Aguilar: Madrid, Spain, 1961. [Google Scholar]
- ACCORD. Diazepam 2 mg/5 mL Oral Solution Sugar Free. Package Leaflet: Information for the Patient. Available online: https://www.medicines.org.uk/emc/product/5783/pil (accessed on 3 August 2020).
- AEMPS. CIMA. Ficha Técnica Paracetamol Level 100 mg/mL Solución Oral EFG. Available online: https://cima.aemps.es/cima/dochtml/ft/68318/FT_68318.html (accessed on 3 August 2020).
- RxList. Diazepam Intensol. Available online: https://www.rxlist.com/diazepam-intensol-drug.htm#side_effects (accessed on 3 August 2020).
- Focus Pharmaceuticals Ltd. Prednisolone 10 mg/mL Oral Solution. Package Leaflet: Information for the User. Available online: https://www.medicines.org.uk/emc/product/3370/smpc (accessed on 3 August 2020).
- PRIMEX PHARMACEUTICALS. Ozalin® 2 mg/mL. Package Leaflet: Information for the User. Available online: https://mri.cts-mrp.eu/Human/Downloads/FR_H_0610_001_FinalPL.pdf (accessed on 4 August 2020).
- AEMPS. CIMA. Ficha Técnica Flumil 20 mg/mL Solución Oral. Available online: https://cima.aemps.es/cima/dochtml/ft/63512/FT_63512.html (accessed on 5 August 2020).
- AEMPS. CIMA. Ficha Técnica Lanacordin Pediátrico 0.05 mg/mL Solución Oral. Available online: https://cima.aemps.es/cima/dochtml/ft/34755/FichaTecnica_34755.html (accessed on 5 August 2020).
- Rosemont Pharmaceuticals Limited. Paracetamol 120 mg/5 mL Oral Suspension. Package Leaflet: Information for the User. Available online: https://www.medicines.org.uk/emc/product/6693/smpc (accessed on 7 August 2020).
- Pinewood Laboratories Limited. Junior Parapaed 120 mg/5 mL Oral Suspension. Patient Information Leaflet. Available online: https://www.hpra.ie/img/uploaded/swedocuments/bf1ca653-1934-4daa-8632-f76ebef7c70c.pdf (accessed on 7 August 2020).
- AEMPS. CIMA. Ficha Técnica Mycostatin 100,000 UI/mL Suspensión Oral. Available online: https://cima.aemps.es/cima/dochtml/ft/28262/FichaTecnica_28262.html (accessed on 7 August 2020).
- AEMPS. CIMA. Ficha Técnica Algidrin Pediatrico 20 mg/mL Suspensión Oral. Available online: https://cima.aemps.es/cima/dochtml/ft/78859/FichaTecnica_78859.html (accessed on 8 August 2020).
- AEMPS. CIMA. Ficha Técnica Septrin Pediatrico 8 mg/40 mg/mL Suspension Oral. Available online: https://cima.aemps.es/cima/pdfs/es/ft/48671/48671_ft.pdf (accessed on 8 August 2020).
- Pinewood Laboratories Limited. Patient Information Leaflet: Paediatric Paracetamol Elixir BP Paracetamol 120 mg/5 mL. Available online: https://www.drugs.com/uk/paediatric-paracetamol-elixir-bp-leaflet.html (accessed on 9 August 2020).
- Aspen Pharma. Lanoxin Pg Elixir. Package Leaflet: Information for the User. Available online: https://www.medicines.org.uk/emc/product/5463/smpc (accessed on 10 August 2020).
- Medicine-Online. Daleron Syrup 120 mg/5 mL. 100 mL. Available online: https://www.medicine-online.org/en/quality-products/2483-daleron-syrup-120-mg-5-ml-100-ml-.html (accessed on 10 August 2020).
- Pinewood Healthcare. Loratadine 5 mg/5 mL Syrup. Patient Information Leaflet. Available online: https://www.medicines.org.uk/emc/product/4567/smpc (accessed on 11 August 2020).
- AEMPS. CIMA. Ficha Técnica Polaramine 0.4 mg/mL Jarabe. Available online: https://cima.aemps.es/cima/dochtml/ft/32801/FichaTecnica_32801.html (accessed on 11 August 2020).
- AEMPS. CIMA. Ficha Técnica Mucosan Pediátrico 3 mg/mL Jarabe. Available online: https://cima.aemps.es/cima/dochtml/ft/56156/FT_56156.html (accessed on 11 August 2020).
- AEMPS. CIMA. Ficha Técnica Romilar 15 mg/mL Gotas Orales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/24379/FT_24379.html (accessed on 12 August 2020).
- AEMPS. CIMA. Ficha Técnica Alerlisin 10 mg/mL Gotas Orales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/62788/FT_62788.html (accessed on 12 August 2020).
- AEMPS. CIMA. Fiche Técnica Cleboril Pediátrico 62.5 µg Gotas Orales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/56657/FichaTecnica_56657.html (accessed on 12 August 2020).
- AEMPS. CIMA. Ficha Técnica Fluor Lacer 1.4 mg/mL Gotas Orales. Available online: https://cima.aemps.es/cima/dochtml/ft/57746/FT_57746.html (accessed on 13 August 2020).
- AEMPS. CIMA. Fiche Técnica Hidropolivit Gotas Orales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/44000/FT_44000.html (accessed on 13 August 2020).
- AEMPS. CIMA. Fiche Técnica Largactil 40 mg/mL Gotas Orales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/23661/FichaTecnica_23661.html (accessed on 13 August 2020).
- AEMPS. CIMA. Ficha Técnica Nemactil 40 mg/mL Gotas Orales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/41573/FT_41573.html (accessed on 14 August 2020).
- AEMPS. CIMA. Fiche Técnica Zamene 22.75 mg/mL Gotas Orales en Suspensión. Available online: https://cima.aemps.es/cima/dochtml/ft/61050/FT_61050.html (accessed on 14 August 2020).
- AEMPS. CIMA. Fiche Técnica Dezacor 22.75 mg/mL Gotas Orales en Suspensión. Available online: https://cima.aemps.es/cima/dochtml/ft/61049/FT_61049.html (accessed on 14 August 2020).
- EMC (ELECTRONIC MEDICINES COMPENDIUM). Atropine Eye Drops BP 1.0% w/v Vistatropine Eye Drops 1.0% w/v. Available online: https://www.medicines.org.uk/emc/medicine/29117 (accessed on 24 August 2020).
- AEMPS. CIMA. Fiche Técnica Chibroxin 3 mg/ml Colirio en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/59248/FichaTecnica_59248.html (accessed on 24 August 2020).
- AEMPS. CIMA. Fiche Técnica Rhinovín® Infantil 0.5 mg/mL Gotas Nasales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/37037/FT_37037.html (accessed on 24 August 2020).
- AEMPS. CIMA. Ficha Técnica Utabon Niños 0.25 mg/mL Gotas Nasales en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/46065/FT_46065.html (accessed on 25 August 2020).
- AEMPS. CIMA. Fiche Técnica Cetraxal Ótico 3 mg/mL Gotas Óticas en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/60883/FT_60883.html (accessed on 25 August 2020).
- AEMPS. CIMA. Fiche Técnica Otix Gotas Óticas en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/59381/FT_59381.html (accessed on 25 August 2020).
- AEMPS. CIMA. Fiche Técnica Ciproxina Simple 3 mg/L Gotas Óticas en Solución. Available online: https://cima.aemps.es/cima/dochtml/ft/64940/FichaTecnica_64940.html (accessed on 25 August 2020).
- AEMPS. CIMA. Fiche Técnica Digoxina Kern Pharma 0.25 mg/mL Solución Inyectable. Available online: https://cima.aemps.es/cima/dochtml/ft/34753/FT_34753.html (accessed on 25 August 2020).
- FDA. Abilify Solution Oral. Medication Guide. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021436s038,021713s030,021729s022,021866s023lbl.pdf (accessed on 10 October 2020).
- FDA. Demerol Solution Oral. Medication Guide. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/005010s058lbl.pdf (accessed on 10 October 2020).
- DAILYMED. DIAZEPAM SOLUTION, Lannett Company. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b8cb8fe7-3744-41fa-8981-05a7fa8a2ec3 (accessed on 16 October 2020).
- FDA. Adzenys ER Suspension, Extended Release; Oral. Medication Guide. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eb1cc8d0-4231-41ea-8535-4fd872129713 (accessed on 16 October 2020).
- DAILYMED. Children’s Tylenol® Cold Plus Cough Plus Sore Throat—Acetaminophen and Dextromethorphan Hydrobromide Suspension. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e83f7911-22b4-4ffc-8f49-68f6acd2ebf5 (accessed on 15 October 2020).
- DAILYMED. Dyanavel XR—Amphetamine Suspension, Extended Release. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a8a7eb93-4192-4826-bbf1-82c06634f553 (accessed on 15 October 2020).
- DAILYMED. Midazolam Hydrochloride Syrup. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0be63f63-6a93-4782-8f9c-d13ca5ae44bd (accessed on 19 October 2020).
- DAILYMED. Ciprofloxacin and Dexamethasone Suspension/Drops. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f07cf7d6-c233-884e-342d-c6c8cd2c8438 (accessed on 19 October 2020).
- DAILYMED. Allergy Eye Drops—Ketotifen Fumarate Solution/Drops. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aa9133fd-166e-4f05-9ca8-851ece692874 (accessed on 19 October 2020).
- DAILYMED. Little Remedies Decongestant Nasal Drops—Phenylephrine Hydrochloride Liquid. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8355eed-f1a2-4b4a-b65f-c2f4b28c12d1 (accessed on 19 October 2020).
- DAILYMED. BIO-G-TUSS PEDIATRIC DROPS—Dextromethorphan HBR, Guaifenesin, Phenylephrine HCL Solution/Drops. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42045759-eaf2-4ffb-8a68-043f661acf8a (accessed on 19 October 2020).
- DAILYMED. Children’s Motrin—Ibuprofen Tablet, Chewable. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=15b0b56d-6188-4937-b541-902022e35b24#footnote-1 (accessed on 15 October 2020).
- DAILYMED. Acetaminophen Children’s—Acetaminophen Tablet, Chewable. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ce4f8cd-4a58-4c92-a8c1-c3cfhttps://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ce4f8cd-4a58-4c92-a8c1-c3cfd591dba2d591dba2 (accessed on 15 October 2020).
- DAILYMED. Diazepam Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f226e5c1-9f6f-4bae-890d-ad4bdaeaf039 (accessed on 16 October 2020).
- DAILYMED. Dexamethasone 1.5 MG—Dexamethasone 1.5 mg Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fcc75691-974e-4a67-b9d7-64dde72b5595 (accessed on 19 October 2020).
- Van Der Vossen, A.C.; Van Der Velde, I.; Smeets, O.; Postma, D.; Eckhardt, M.; Vermes, A.; Koch, B.C.P.; Vulto, A.; Hanff, L. Formulating a poorly water soluble drug into an oral solution suitable for paediatric patients; lorazepam as a model drug. Eur. J. Pharm. Sci. 2017, 100, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vossen, A.; Van Der Velde, I.; Smeets, O.; Postma, D.; Vermes, A.; Koch, B.; Vulto, A.; Hanff, L. Design and stability study of an oral solution of amlodipine besylate for pediatric patients. Eur. J. Pharm. Sci. 2016, 92, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Gobetti, C.; Balsan, M.E.; Ayres, M.V.; De Almeida, S.H.O.; Simon, E.D.S.; Wingert, N.R.; Oppe, T.P.; Garcia, C.V. Development and Stability Control of Pediatric Oral Tizanidine Hydrochloride Formulations for Hospital Use. AAPS PharmSciTech 2020, 21, 1–11. [Google Scholar] [CrossRef]
- De Goede, A.; Boedhram, R.; Eckhardt, M.; Hanff, L.; Koch, B.; Vermaat, C.; Vermes, A. Development and validation of a paediatric oral formulation of clonidine hydrochloride. Int. J. Pharm. 2012, 433, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Merino-Bohórquez, V.; Delgado-Valverde, M.; García-Palomo, M.; Dávila-Pousa, M.; Cañete, C.; Villaronga, M.; Rodriguez-Marrodán, B.; López-Rojas, R.; Cameán-Fernández, M.; The Pharmaceutical Technology Working Group and Pediatric Pharmacy Working Group of the Spanish Society of Hospital Pharmacy (SEFH). Physicochemical and microbiological stability of two new oral liquid formulations of clonidine hydrochloride for pediatric patients. Pharm. Dev. Technol. 2018, 24, 465–478. [Google Scholar] [CrossRef]
- Lopalco, A.; Curci, A.; Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Franco, M.; Denora, N. Pharmaceutical preformulation studies and paediatric oral formulations of sodium dichloroacetate. Eur. J. Pharm. Sci. 2019, 127, 339–350. [Google Scholar] [CrossRef]
- Provenza, N.; Calpena, A.; Mallandrich, M.; Sanchez, A.; Egea, M.; Clares, B. Permeation studies through porcine small intestine of furosemide solutions for personalised paediatric administration. Int. J. Pharm. 2014, 475, 208–213. [Google Scholar] [CrossRef]
- Kini, R.S.; Bhilegaonkar, P.S. Pharmaceutical Formulation Comprising Paracetamol Especially for Pediatrics. IN Patent 201,721,007,863, 7 March 2017. [Google Scholar]
- Santoveña, A.; Sánchez-Negrín, E.; Charola, L.; Llabrés, M.; Farina, J.B. Study of quality and stability of ursodeoxycholic acid formulations for oral pediatric administration. Int. J. Pharm. 2014, 477, 32–38. [Google Scholar] [CrossRef]
- Santoveña, A.; Hernández-Paiz, Z.; Fariña, J. Design of a pediatric oral formulation with a low proportion of hydrochlorothiazide. Int. J. Pharm. 2012, 423, 360–364. [Google Scholar] [CrossRef]
- Alayoubi, A.; Daihom, B.; Adhikari, H.; Mishra, S.R.; Helms, R.; Almoazen, H. Development of a taste-masked oral suspension of clindamycin HCl using ion exchange resin Amberlite IRP 69 for use in pediatrics. Drug Dev. Ind. Pharm. 2016, 42, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Goode, G.A.; Wagh, S.J.; Irby, D.J.; Ma, D.; Jacobs, R.F.; Kearns, G.L.; Almoazen, H. Bioavailability testing of a newly developed clindamycin oral suspension in a pediatric porcine model. Pharm. Dev. Technol. 2019, 24, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Suthar, A.M.; Patel, M.M. Suspension of Isoniazid Formulated Using Cationic Resin for Pediatric Use. Int. J. Curr. Pharm. Res. 2014, 6, 6–10. Available online: https://pdfs.semanticscholar.org/8c82/227ce826c2a063af50d4e90051a52ceb5285.pdf (accessed on 2 December 2020).
- Bhuva, F.; Patel, L. Pediatric Nasal Spray Solution: Factorial Design Development and Evaluation. J. Drug Deliv. Ther. 2018, 8, 355–365. [Google Scholar] [CrossRef]


| Name | Website | Creator |
|---|---|---|
| ACToR —Aggregated Computational Toxicology Resource | www.actor.epa.gov/actor/home.xhtml (accessed on 15 November 2020) | US Environmental Protection Agency’s (EPA) National Center for Computational Toxicology (NCCT) |
| STEP—Safety and Toxicity of Excipients for Paediatrics * | www.eupfi.org/step-database-info/ (accessed on 15 November 2020) | European Paediatric Formulation Initiative |
| TOXNET—Toxicology Data Network | www.nlm.nih.gov/toxnet/index.html (accessed on 15 November 2020) | Specialized Information Services (SIS) USA |
| Vitic | www.lhasalimited.org/products/vitic.htm (accessed on 2 November 2020) | Lhasa Limited |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the Paediatric Population: A Review. Pharmaceutics 2021, 13, 387. https://doi.org/10.3390/pharmaceutics13030387
Rouaz K, Chiclana-Rodríguez B, Nardi-Ricart A, Suñé-Pou M, Mercadé-Frutos D, Suñé-Negre JM, Pérez-Lozano P, García-Montoya E. Excipients in the Paediatric Population: A Review. Pharmaceutics. 2021; 13(3):387. https://doi.org/10.3390/pharmaceutics13030387
Chicago/Turabian StyleRouaz, Khadija, Blanca Chiclana-Rodríguez, Anna Nardi-Ricart, Marc Suñé-Pou, Dèbora Mercadé-Frutos, Josep María Suñé-Negre, Pilar Pérez-Lozano, and Encarna García-Montoya. 2021. "Excipients in the Paediatric Population: A Review" Pharmaceutics 13, no. 3: 387. https://doi.org/10.3390/pharmaceutics13030387