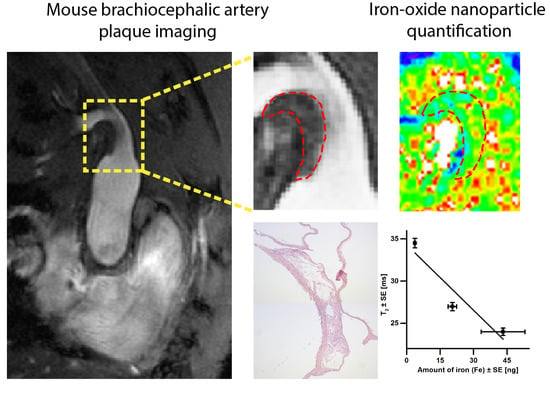
Iron Oxide Nanoparticle Uptake in Mouse Brachiocephalic Artery Atherosclerotic Plaque Quantified by T2-Mapping MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. MRI Protocol
2.3. MRI Data Analysis
2.4. Histology and Immunohistochemistry
2.5. pEPR
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quillard, T.; Libby, P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ. Res. 2012, 111, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; De Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the Vulnerable Plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasterkamp, G.; Schoneveld, A.H.; Van Der Wal, A.C.; Haudenschild, C.C.; Clarijs, R.J.; Becker, A.E.; Hillen, B.; Borst, C. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: The remodeling paradox. J. Am. Coll. Cardiol. 1998, 32, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nat. Cell Biol. 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Swirski, F.K.; Pittet, M.J.; Kircher, M.F.; Aikawa, E.; Jaffer, F.A.; Libby, P.; Weissleder, R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl. Acad. Sci. USA 2006, 103, 10340–10345. [Google Scholar] [CrossRef] [Green Version]
- Ruehm, S.G.; Corot, C.; Vogt, P.; Kolb, S.; Debatin, J.F. Magnetic Resonance Imaging of Atherosclerotic Plaque With Ultrasmall Superparamagnetic Particles of Iron Oxide in Hyperlipidemic Rabbits. Circulation 2001, 103, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Korosoglou, G.; Weiss, R.G.; Kedziorek, D.A.; Walczak, P.; Gilson, W.D.; Schär, M.; Sosnovik, D.E.; Kraitchman, D.L.; Boston, R.C.; Bulte, J.W.; et al. Noninvasive Detection of Macrophage-Rich Atherosclerotic Plaque in Hyperlipidemic Rabbits Using “Positive Contrast” Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2008, 52, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Kooi, M.E.; Cappendijk, V.C.; Cleutjens, K.B.J.M.; Kessels, A.G.H.; Kitslaar, P.J.E.H.M.; Borgers, M.; Frederik, P.M.; Daemen, M.J.A.P.; Van Engelshoven, J.M.A. Accumulation of Ultrasmall Superparamagnetic Particles of Iron Oxide in Human Atherosclerotic Plaques Can Be Detected by In Vivo Magnetic Resonance Imaging. Circulation 2003, 107, 2453–2458. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.Y.; Muller, K.H.; Graves, M.J.; Li, Z.Y.; Walsh, S.R.; Young, V.; Sadat, U.; Howarth, S.P.; Gillard, J.H. Iron Oxide Particles for Atheroma Imaging. Arter. Thromb. Vasc. Biol. 2009, 29, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, R.A.; Mallawarachi, C.; U-King-Im, J.-M.; Graves, M.J.; Horsley, J.; Goddard, M.J.; Brown, A.; Wang, L.; Kirkpatrick, P.J.; Brown, J.; et al. Identifying Inflamed Carotid Plaques Using In Vivo USPIO-Enhanced MR Imaging to Label Plaque Macrophages. Arter. Thromb. Vasc. Biol. 2006, 26, 1601–1606. [Google Scholar] [CrossRef] [Green Version]
- Sigovan, M.; Kaye, E.; Lancelot, E.; Corot, C.; Provost, N.; Majd, Z.; Breisse, M.; Canet-Soulas, E. Anti-Inflammatory Drug Evaluation in ApoE−/− Mice by Ultrasmall Superparamagnetic Iron Oxide–Enhanced Magnetic Resonance Imaging. Investig. Radiol. 2012, 47, 546–552. [Google Scholar] [CrossRef]
- Sigovan, M.; Bessaad, A.; Alsaid, H.; Lancelot, E.; Corot, C.; Neyran, B.; Provost, N.; Majd, Z.; Breisse, M.; Canet-Soulas, E. Assessment of Age Modulated Vascular Inflammation in ApoE−/− Mice by USPIO-Enhanced Magnetic Resonance Imaging. Investig. Radiol. 2010, 45, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.R.; Varma, G.; Wiethoff, A.J.; Smith, A.; Mattock, K.; Jansen, C.H.; Warley, A.; Taupitz, M.; Schaeffter, T.; Botnar, R.M. Noninvasive Assessment of Atherosclerotic Plaque Progression in ApoE−/− Mice Using Susceptibility Gradient Mapping. Circ. Cardiovasc. Imaging 2011, 4, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Sadat, U.; Howarth, S.P.; Usman, A.; Tang, T.Y.; Graves, M.J.; Gillard, J.H. Sequential Imaging of Asymptomatic Carotid Atheroma Using Ultrasmall Superparamagnetic Iron Oxide–enhanced Magnetic Resonance Imaging: A Feasibility Study. J. Stroke Cerebrovasc. Dis. 2013, 22, e271–e276. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Tang, T.Y.; Graves, M.J.; Müller, K.H.; Gillard, J.H. In vivo carotid plaque MRI using quantitative T 2 * measurements with ultrasmall superparamagnetic iron oxide particles: A dose-response study to statin therapy. NMR Biomed. 2010, 24, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.Y.; Howarth, S.P.; Miller, S.R.; Graves, M.J.; Patterson, A.J.; U-King-Im, J.-M.; Li, Z.Y.; Walsh, S.R.; Brown, A.P.; Kirkpatrick, P.J.; et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. J. Am. Coll. Cardiol. 2009, 53, 2039–2050. [Google Scholar] [CrossRef]
- Degnan, A.J.; Patterson, A.J.; Tang, T.Y.; Howarth, S.P.; Gillard, J.H. Evaluation of Ultrasmall Superparamagnetic Iron Oxide-Enhanced MRI of Carotid Atherosclerosis to Assess Risk of Cerebrovascular and Cardiovascular Events: Follow-Up of the ATHEROMA Trial. Cerebrovasc. Dis. 2012, 34, 169–173. [Google Scholar] [CrossRef]
- Sadat, U.; Usman, A.; Gillard, J.H. Imaging pathobiology of carotid atherosclerosis with ultrasmall superparamagnetic particles of iron oxide. Curr. Opin. Cardiol. 2017, 32, 437–440. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.H.; Schoormans, J.; Stiekema, L.C.; Calcagno, C.; Cicha, I.; Alexiou, C.; Strijkers, G.J.; Nederveen, A.J.; Stroes, E.S.; Coolen, B.F. Plaque Permeability Assessed With DCE-MRI Associates With USPIO Uptake in Patients With Peripheral Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 2081–2083. [Google Scholar] [CrossRef]
- Smits, L.P.; Tiessens, F.; Zheng, K.H.; Stroes, E.S.; Nederveen, A.J.; Coolen, B.F. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 2017, 263, 211–218. [Google Scholar] [CrossRef]
- Ruetten, P.P.R.; Cluroe, A.D.; Usman, A.; Priest, A.N.; Gillard, J.H.; Graves, M.J. Simultaneous MRI water-fat separation and quantitative susceptibility mapping of carotid artery plaque pre- and post-ultrasmall superparamagnetic iron oxide-uptake. Magn. Reson. Med. 2020, 84, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, M.; Biasiolli, L.; Akbar, N.; Galassi, F.; Chai, J.T.; Robson, M.D.; Choudhury, R.P. T2 mapping MRI technique quantifies carotid plaque lipid, and its depletion after statin initiation, following acute myocardial infarction. Atherosclerosis 2018, 279, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Biasiolli, L.; Lindsay, A.C.; Chai, J.T.; Choudhury, R.P.; Robson, M.D. In-vivo quantitative T2 mapping of carotid arteries in atherosclerotic patients: Segmentation and T2 measurement of plaque components. J. Cardiovasc. Magn. Reson. 2013, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Biasiolli, L.; Chai, J.T.; Li, L.; Handa, A.; Jezzard, P.; Choudhury, R.; Robson, M.D. Histological validation of carotid plaque characterization by in-vivo T2 mapping in patients with recent cerebrovascular events: Preliminary results. J. Cardiovasc. Magn. Reson. 2014, 16, P173. [Google Scholar] [CrossRef] [Green Version]
- Heeneman, S. Control of atherosclerotic plaque vulnerability: Insights from transgenic mice. Front. Biosci. 2008, 13, 6289–6313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutgens, E.; Daemen, M.; Kockx, M.; Doevendans, P.; Hofker, M.; Havekes, L.; Wellens, H.; De Muinck, E.D. Atherosclerosis in APOE*3-Leiden Transgenic Mice. Circulation 1999, 99, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Coolen, B.F.; Simonis, F.F.; Geelen, T.; Moonen, R.P.; Arslan, F.; Paulis, L.E.; Nicolay, K.; Strijkers, G.J. Quantitative T2mapping of the mouse heart by segmented MLEV phase-cycled T2preparation. Magn. Reson. Med. 2013, 72, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Torfs, G.; Vandewege, J.; Bauwelinck, J.; Verbiest, J.R. Sensitive and quantitative pEPR detection system for SPIO nanoparticles. Electron. Lett. 2013, 49, 1600–1601. [Google Scholar] [CrossRef]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Lévy, M.; Wilhelm, C.; Devaud, M.; Levitz, P.; Gazeau, F. How cellular processing of superparamagnetic nanoparticles affects their magnetic behavior and NMR relaxivity. Contrast Media Mol. Imaging 2012, 7, 373–383. [Google Scholar] [CrossRef]
- Tanimoto, A.; Oshio, K.; Suematsu, M.; Pouliquen, D.; Stark, D.D. Relaxation effects of clustered particles. J. Magn. Reson. Imaging 2001, 14, 72–77. [Google Scholar] [CrossRef]
- Hak, S.; Goa, P.E.; Stenmark, S.; Bjerkholt, F.F.; Haraldseth, O. Transverse relaxivity of iron oxide nanocrystals clustered in nanoemulsions: Experiment and theory. Magn. Reson. Med. 2014, 74, 858–867. [Google Scholar] [CrossRef]
- Van Heeswijk, R.B.; Piccini, D.; Feliciano, H.; Hullin, R.; Schwitter, J.; Stuber, M. Self-navigated isotropic three-dimensional cardiac T2 mapping. Magn. Reson. Med. 2015, 73, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Baeßler, B.; Schaarschmidt, F.; Stehning, C.; Schnackenburg, B.; Giolda, A.; Maintz, D.; Bunck, A.C. Reproducibility of three different cardiac T 2 -mapping sequences at 1.5T. J. Magn. Reson. Imaging 2016, 44, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Coyne, D.W. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert Opin. Pharmacother. 2009, 10, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Spinowitz, B.S.; Kausz, A.T.; Baptista, J.; Noble, S.D.; Sothinathan, R.; Bernardo, M.V.; Brenner, L.; Pereira, B.J.G. Ferumoxytol for Treating Iron Deficiency Anemia in CKD. J. Am. Soc. Nephrol. 2008, 19, 1599–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancy, A.D.; Olzinski, A.R.; Hu, T.C.-C.; Lenhard, S.C.; Aravindhan, K.; Gruver, S.M.; Jacobs, P.M.; Willette, R.N.; Jucker, B.M. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: Critical determinants of atherosclerotic plaque labeling. J. Magn. Reson. Imaging 2005, 21, 432–442. [Google Scholar] [CrossRef]
- Herborn, C.U.; Vogt, F.M.; Lauenstein, T.C.; Dirsch, O.; Corot, C.; Robert, P.; Ruehm, S.G. Magnetic resonance imaging of experimental atherosclerotic plaque: Comparison of two ultrasmall superparamagnetic particles of iron oxide. J. Magn. Reson. Imaging 2006, 24, 388–393. [Google Scholar] [CrossRef]
- Alam, S.R.; Shah, A.S.; Richards, J.M.J.; Lang, N.N.; Barnes, G.R.; Joshi, N.; MacGillivray, T.; McKillop, G.; Mirsadraee, S.; Payne, J.; et al. Ultrasmall Superparamagnetic Particles of Iron Oxide in Patients With Acute Myocardial Infarction. Circ. Cardiovasc. Imaging 2012, 5, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, Y.; Tachibana, A.; Heidary, S.; Yang, P.C.; McConnell, M.V.; Dash, R. Ferumoxytol-enhanced cardiovascular magnetic resonance detection of early stage acute myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Patterson, A.J.; Yuan, J.; Cluroe, A.; Patterson, I.; Graves, M.J.; Gillard, J.H.; Sadat, U. Ferumoxytol-enhanced three-dimensional magnetic resonance imaging of carotid atheroma- a feasibility and temporal dependence study. Sci. Rep. 2020, 10, 1808. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moonen, R.P.M.; Coolen, B.F.; Sluimer, J.C.; Daemen, M.J.A.P.; Strijkers, G.J. Iron Oxide Nanoparticle Uptake in Mouse Brachiocephalic Artery Atherosclerotic Plaque Quantified by T2-Mapping MRI. Pharmaceutics 2021, 13, 279. https://doi.org/10.3390/pharmaceutics13020279
Moonen RPM, Coolen BF, Sluimer JC, Daemen MJAP, Strijkers GJ. Iron Oxide Nanoparticle Uptake in Mouse Brachiocephalic Artery Atherosclerotic Plaque Quantified by T2-Mapping MRI. Pharmaceutics. 2021; 13(2):279. https://doi.org/10.3390/pharmaceutics13020279
Chicago/Turabian StyleMoonen, Rik P. M., Bram F. Coolen, Judith C. Sluimer, Mat J. A. P. Daemen, and Gustav J. Strijkers. 2021. "Iron Oxide Nanoparticle Uptake in Mouse Brachiocephalic Artery Atherosclerotic Plaque Quantified by T2-Mapping MRI" Pharmaceutics 13, no. 2: 279. https://doi.org/10.3390/pharmaceutics13020279