Transmucosal Solid Lipid Nanoparticles to Improve Genistein Absorption via Intestinal Lymphatic Transport
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
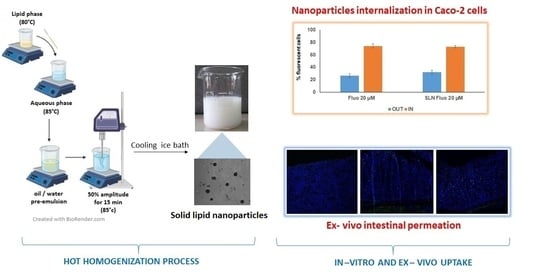
2.2. Preparation of Unloaded SLN
2.3. Preparation of GEN-Loaded SLN
2.4. Analysis of Particle Size, Polydispersity Index, and Physical Stability
2.5. Determination of GEN Loading
2.6. Morphological Analysis and Zeta Potential
2.7. In Vitro GEN Release Study
2.8. Evaluation of SLN Characteristics Useful for Intestinal Lymphatic Transport
2.8.1. In Vitro Chylomicron-Like Structure Formation
2.8.2. Ex Vivo Uptake Study on Intestinal Mucosa
2.8.3. In Vitro Cellular Uptake Study
2.9. Statistical Analysis
3. Results and Discussions
3.1. Preparation of GEN-Loaded SLN
3.2. GEN Loading Capability
3.3. Analysis of Particle Size, Polydispersity Index, and Physical Stability
3.4. Morphological Analysis and Zeta Potential
3.5. Determination of GEN Loading
3.6. In Vitro Drug Release Studies
3.7. Evaluation of SLN Characteristics Useful for Intestinal Lymphatic Transport
3.7.1. In Vitro Chylomicron-Like Structure Formation
3.7.2. Ex Vivo Uptake Study on Intestinal Mucosa
3.7.3. In Vitro Cellular Uptake Study
4. Conclusions
Supplementary Materials
Artwork
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishioka, Y.; Yoshino, H. Lymphatic Targeting with Nanoparticulate System. Adv. Drug. Deliv. Rev. 2001, 47, 55–64. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Lee, Y.-B. Nano-Sized Drug Delivery Systems for Lymphatic Delivery. J. Nanosci. Nanotechnol. 2014, 14, 868–880. [Google Scholar] [CrossRef]
- Darwis, Y.; Ali Khan, A.; Mudassir, J.; Mohtar, N. Advanced Drug Delivery to the Lymphatic System: Lipid-Based Nanoformulations. Int. J. Nanomed. 2013, 2733–2744. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Vuddanda, P.R.; Singh, S. Intestinal Lymphatic Delivery of Praziquantel by Solid Lipid Nanoparticles: Formulation Design, In Vitro and In Vivo Studies. J. Nanotechnol. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Yang, Q.; Bagby, T.R.; Forrest, M.L. Lymphatic Drug Delivery Using Engineered Liposomes and Solid Lipid Nanoparticles. Adv. Drug. Deliv. Rev. 2011, 63, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.; Garg, T.; Murthy, R.S.R.; Rath, G.; Goyal, A.K. Recent Approaches of Lipid-Based Delivery System for Lymphatic Targeting via Oral Route. J. Drug Target 2014, 22, 871–882. [Google Scholar] [CrossRef]
- Jose, S.; Souto, E.B. Lopinavir Loaded Solid Lipid Nanoparticles (SLN) for Intestinal Lymphatic Targeting. Eur. J. Pharm. Sci. 2011, 42, 11–18. [Google Scholar] [CrossRef]
- Krishnan, Y.; Mukundan, S.; Akhil, S.; Gupta, S.; Viswanad, V. Enhanced Lymphatic Uptake of Leflunomide Loaded Nanolipid Carrier via Chylomicron Formation for the Treatment of Rheumatoid Arthritis. Adv. Pharm. Bull. 2018, 8, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y. Mechanisms of Cancer Chemoprevention by Soy Isoflavone Genistein. Cancer Met. Rev. 2002, 21, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [Green Version]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Sikand, G.; Kris-Etherton, P.; Boulos, N.M. Impact of Functional Foods on Prevention of Cardiovascular Disease and Diabetes. Curr. Cardiol. Rep. 2015, 17, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M. Effects of Genistein and Hormone-Replacement Therapy on Bone Loss in Early Postmenopausal Women: A Randomized Double-Blind Placebo-Controlled Study. J. Bone Min. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef]
- Ma, W.; Yuan, L.; Yu, H.; Ding, B.; Xi, Y.; Feng, J.; Xiao, R. Genistein as a Neuroprotective Antioxidant Attenuates Redox Imbalance Induced by Β-amyloid Peptides 25–35 in PC12 Cells. Int. J. Dev. Neurosci. 2010, 28, 289–295. [Google Scholar] [CrossRef]
- Langasco, R.; Fancello, S.; Rassu, G.; Cossu, M.; Cavalli, R.; Galleri, G.; Giunchedi, P.; Migheli, R.; Gavini, E. Increasing Protective Activity of Genistein by Loading into Transfersomes: A New Potential Adjuvant in the Oxidative Stress-Related Neurodegenerative Diseases? Phytomedicine 2019, 52, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Porcu, E.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal Delivery of Genistein-Loaded Nanoparticles as a Potential Preventive System against Neurodegenerative Disorders. Pharmaceutics 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aditya, N.P.; Shim, M.; Lee, I.; Lee, Y.; Im, M.-H.; Ko, S. Curcumin and Genistein Coloaded Nanostructured Lipid Carriers: In Vitro Digestion and Antiprostate Cancer Activity. J. Agric. Food Chem. 2013, 61, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.M.M.; Elnaggar, Y.S.R.; Galal, S.; Abdallah, O.Y. Self-Emulsifying Phospholipid Pre-Concentrates (SEPPs) for Improved Oral Delivery of the Anti-Cancer Genistein: Development, Appraisal and Ex-Vivo Intestinal Permeation. Int. J. Pharm. 2016, 511, 745–756. [Google Scholar] [CrossRef]
- Tyagi, N.; Song, Y.H.; De, R. Recent Progress on Biocompatible Nanocarrier-Based Genistein Delivery Systems in Cancer Therapy. J. Drug Target 2019, 27, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Motlekar, N.; Khan, M.A.; Youan, B.-B.C. Preparation and Characterization of Genistein Containing Poly(Ethylene Glycol) Microparticles. J. Appl. Polym. Sci. 2006, 101, 2070–2078. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kim, S.Y.; Ha, K.W.; Kang, M.J.; Huh, J.S.; Im, T.J.; Kim, Y.M.; Park, Y.M.; Kang, K.H.; Lee, S.; et al. Pharmaceutical Evaluation of Genistein-Loaded Pluronic Micelles for Oral Delivery. Arch. Pharm. Res. 2007, 30, 1138–1143. [Google Scholar] [CrossRef]
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit Nanoparticles Containing Genistein: Formulation, Development, and Bioavailability Assessment. Int. J. Nanomed. 2011, 2429–2435. [Google Scholar] [CrossRef] [Green Version]
- Trevaskis, N.L.; Charman, W.N.; Porter, C.J.H. Lipid-Based Delivery Systems and Intestinal Lymphatic Drug Transport: A Mechanistic Update. Adv. Drug. Deliv. Rev. 2008, 60, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Weyhers, H.; Ehlers, S.; Hahn, H.; Souto, E.B.; Müller, R.H. Solid Lipid Nanoparticles (SLN)--Effects of Lipid Composition on in Vitro Degradation and in Vivo Toxicity. Pharmaceutical 2006, 61, 539–544. [Google Scholar]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from Artichoke Heads (Cynara Cardunculus (L.) Subsp. Scolymus Hayek): In Vitro Bio-Accessibility, Intestinal Uptake and Bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef]
- Failla, M.L.; Chitchumronchokchai, C.; Ferruzzi, M.G.; Goltz, S.R.; Campbell, W.W. Unsaturated Fatty Acids Promote Bioaccessibility and Basolateral Secretion of Carotenoids and α-Tocopherol by Caco-2 Cells. Food Funct. 2014, 5, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- de Zampieri, A.L.T.C.; Ferreira, F.S.; Resende, É.C.; Gaeti, M.P.N.; Diniz, D.G.A.; Taveira, S.F.; Lima, E.M. Biodegradable Polymeric Nanocapsules Based on Poly(DL-Lactide) for Genistein Topical Delivery: Obtention, Characterization and Skin Permeation Studies. J. Biomed. Nanotechnol. 2013, 9, 527–534. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Tong, Z.; Kong, X.; Yao, J. Preparation of Chitosan-Based Multifunctional Nanocarriers Overcoming Multiple Barriers for Oral Delivery of Insulin. Mater Sci. Eng. C 2017, 70, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Spada, G.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid Lipid Nanoparticles with and without Hydroxypropyl-β-Cyclodextrin: A Comparative Study of Nanoparticles Designed for Colonic Drug Delivery. Nanotechnology 2012, 23, 095101. [Google Scholar] [CrossRef]
- Burrai, G.P.; Tanca, A.; Cubeddu, T.; Abbondio, M.; Polinas, M.; Addis, M.F.; Antuofermo, E. A First Immunohistochemistry Study of Transketolase and Transketolase-like 1 Expression in Canine Hyperplastic and Neoplastic Mammary Lesions. BMC Vet. Res. 2016, 13, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Obinu, A.; Porcu, E.P.; Piras, S.; Ibba, R.; Carta, A.; Molicotti, P.; Migheli, R.; Dalpiaz, A.; Ferraro, L.; Rassu, G.; et al. Solid Lipid Nanoparticles as Formulative Strategy to Increase Oral Permeation of a Molecule Active in Multidrug-Resistant Tuberculosis Management. Pharmaceutics 2020, 12, 1132. [Google Scholar] [CrossRef]
- Vitiello, V.; Burrai, G.P.; Agus, M.; Anfossi, A.G.; Alberti, A.; Antuofermo, E.; Rocca, S.; Cubeddu, T.; Pirino, S. Ovis Aries Papillomavirus 3 in Ovine Cutaneous Squamous Cell Carcinoma. Vet. Pathol. 2017, 54, 775–782. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug. Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Abdelbary, G.; Fahmy, R.H. Diazepam-Loaded Solid Lipid Nanoparticles: Design and Characterization. AAPS PharmSciTech 2009, 10, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.-J.; Kim, C.-K. Formulation Parameters Determining the Physicochemical Characteristics of Solid Lipid Nanoparticles Loaded with All-Trans Retinoic Acid. Int. J. Pharm. 2002, 243, 135–146. [Google Scholar] [CrossRef]
- Harivardhan Reddy, L.; Murthy, R.S.R. Etoposide-Loaded Nanoparticles Made from Glyceride Lipids: Formulation, Characterization, in Vitro Drug Release, and Stability Evaluation. AAPS PharmSciTech 2005, 6, E158–E166. [Google Scholar] [CrossRef]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and Characterization of Curcuminoids Loaded Solid Lipid Nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Barua, S.; Kim, H.; Hong, S.-C.; Yoo, S.-Y.; Jeon, H.; Cho, Y.; Gil, S.; Oh, K.; Lee, J. Absorption Study of Genistein Using Solid Lipid Microparticles and Nanoparticles: Control of Oral Bioavailability by Particle Sizes. Biomol. Ther. 2017, 25, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Dahan, A.; Hoffman, A. Evaluation of a Chylomicron Flow Blocking Approach to Investigate the Intestinal Lymphatic Transport of Lipophilic Drugs. Eur. J. Pharm. Sci. 2005, 24, 381–388. [Google Scholar] [CrossRef]
- Rangaraj, N.; Pailla, S.R.; Shah, S.; Prajapati, S.; Sampathi, S. QbD Aided Development of Ibrutinib-Loaded Nanostructured Lipid Carriers Aimed for Lymphatic Targeting: Evaluation Using Chylomicron Flow Blocking Approach. Drug. Deliv. Transl. Res. 2020, 10, 1476–1494. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Kristensen, M.; Larsen, F.H.; Astrup, A.; Engelsen, S.B. High Throughput Prediction of Chylomicron Triglycerides in Human Plasma by Nuclear Magnetic Resonance and Chemometrics. Nutr. Metab. 2010, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Hilmer, S.N.; Cogger, V.C.; Fraser, R.; McLean, A.J.; Sullivan, D.; Le Couteur, D.G. Age-Related Changes in the Hepatic Sinusoidal Endothelium Impede Lipoprotein Transfer in the Rat. Hepatol. Baltim. Md. 2005, 42, 1349–1354. [Google Scholar] [CrossRef]
- Bhandari, R.; Kaur, I.P. Pharmacokinetics, Tissue Distribution and Relative Bioavailability of Isoniazid-Solid Lipid Nanoparticles. Int. J. Pharm. 2013, 441, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Karn, P.R.; Cho, W.; Park, H.J.; Park, J.S.; Hwang, S.J. Characterization and Stability Studies of a Novel Liposomal Cyclosporin A Prepared Using the Supercritical Fluid Method: Comparison with the Modified Conventional Bangham Method. Int. J. Nanomed. 2013, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Di Guardo, G. Lipofuscin, Lipofuscin-like Pigments and Autofluorescence. Eur. J. Histochem. EJH 2015, 59, 2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Sample | Compritol 888 ATO (% w/v) § | Tween 80 (% w/v) § | Genistein (% w/v) § |
|---|---|---|---|
| G-SLN a | 2 | 0.5 | 0.02 |
| G-SLN b | 2 | 0.5 | 0.03 |
| G-SLN c | 2 | 0.5 | 0.04 |
| G-SLN d | 2 | 0.5 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obinu, A.; Burrai, G.P.; Cavalli, R.; Galleri, G.; Migheli, R.; Antuofermo, E.; Rassu, G.; Gavini, E.; Giunchedi, P. Transmucosal Solid Lipid Nanoparticles to Improve Genistein Absorption via Intestinal Lymphatic Transport. Pharmaceutics 2021, 13, 267. https://doi.org/10.3390/pharmaceutics13020267
Obinu A, Burrai GP, Cavalli R, Galleri G, Migheli R, Antuofermo E, Rassu G, Gavini E, Giunchedi P. Transmucosal Solid Lipid Nanoparticles to Improve Genistein Absorption via Intestinal Lymphatic Transport. Pharmaceutics. 2021; 13(2):267. https://doi.org/10.3390/pharmaceutics13020267
Chicago/Turabian StyleObinu, Antonella, Giovanni Pietro Burrai, Roberta Cavalli, Grazia Galleri, Rossana Migheli, Elisabetta Antuofermo, Giovanna Rassu, Elisabetta Gavini, and Paolo Giunchedi. 2021. "Transmucosal Solid Lipid Nanoparticles to Improve Genistein Absorption via Intestinal Lymphatic Transport" Pharmaceutics 13, no. 2: 267. https://doi.org/10.3390/pharmaceutics13020267