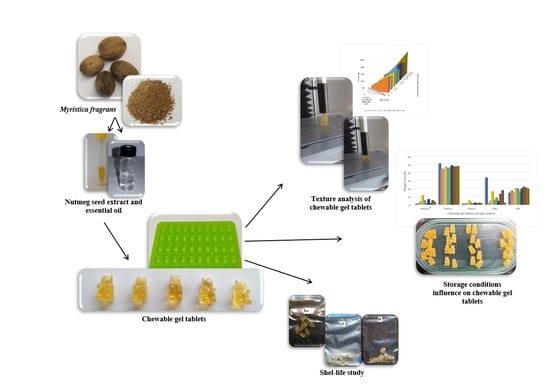
The Effect of Myristica fragrans on Texture Properties and Shelf-Life of Innovative Chewable Gel Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thyme Syrup Preparation
2.3. Nutmeg Seed Products Preparation
2.4. Chewable Gel Tablets’ Preparation and Compositions
2.5. Chewable Gel Tablets’ Physical Parameters: Mass Change, Firmness and Springiness
2.6. Chewable Gel Tablets’ Quality Determination
2.7. Microbial Contamination of Equipment
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chewable Gel Tablets’ Composition
3.2. Chewable Gel Tablets’ Weight Variation and Quality
3.3. Chewable Gel Tablets’ Firmness and Springiness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nyamweya, N.N.; Kimani, S.N. Chewable Tablets: A Review of Formulation Considerations. Pharm. Technol. 2020, 44, 38–44. [Google Scholar]
- Taranum, R.; Mittapally, S. Soft Chewable Drug Delivery System: Oral Medicated Jelly and Soft Chew. J. Drug Deliv. Ther. 2018, 8, 65–72. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines; World Health Organization: Genève, Switzerland, 2019; pp. 1–6. [Google Scholar]
- Čižauskaitė, U.; Jakubaitytė, G.; Žitkevičius, V.; Kasparavičienė, G. Natural Ingredients-Based Gummy Bear Composition Designed According to Texture Analysis and Sensory Evaluation in Vivo. Molecules 2019, 24, 1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMars, L.L.; Ziegler, G.R. Texture and Structure of Gelatin/Pectin-Based Gummy Confections. Food Hydrocoll. 2001, 15, 643–653. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Tablets Compressi. In European Pharmacopoeia 10.3; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 937–939. [Google Scholar]
- Bhusnure, O.G.; Shaikh, F.E.; Sugave, B.K.; Kavale, B.S.; Sayyed, R.A.; Hucche, B.S. Formulation Strategies for Taste-Masking of Chewable Tablets. Indo Am. J. Pharm. Res. 2015, 5, 3836–3849. [Google Scholar]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of Antimicrobial Gummy Candies with Addition of Bovine Colostrum, Essential Oils and Probiotics. Int. J. Food Sci. Technol. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- Wright, D.J.; Potter, J.F.; Clark, A.; Blyth, A.; Maskrey, V.; Mencarelli, G.; Wicks, S.O.; Craig, D.Q.M. Administration of Aspirin Tablets Using a Novel Gel-Based Swallowing Aid: An Open-Label Randomised Controlled Cross-over Trial. BMJ Innov. 2019, 5, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Schrieber, R.; Gareis, H. Gelatine Handbook. Theory and Industrial Practice; WILEY-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2007. [Google Scholar]
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Pinson, M.L.; Waibel, K.H. Safe Administration of a Gelatin-Containing Vaccine in an Adult with Galactose-α-1,3-Galactose Allergy. Vaccine 2015, 33, 1231–1232. [Google Scholar] [CrossRef]
- Michon, C.; Cuvelier, G.; Launay, B. Concentration Dependence of the Critical Viscoelastic Properties of Gelatin at the Gel Point. Rheol. Acta 1993, 32, 94–103. [Google Scholar] [CrossRef]
- Rivero, R.; Archaina, D.; Sosa, N.; Leiva, G.; Baldi Coronel, B.; Schebor, C. Development of Healthy Gummy Jellies Containing Honey and Propolis. J. Sci. Food Agric. 2020, 100, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery Gels: Effects of Low Calorie Sweeteners on the Rheological Properties and Microstructure of Fish Gelatin. Food Hydrocoll. 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Tong, Q.; Xiao, Q.; Lim, L.T. Effects of Glycerol, Sorbitol, Xylitol and Fructose Plasticisers on Mechanical and Moisture Barrier Properties of Pullulan-Alginate-Carboxymethylcellulose Blend Films. Int. J. Food Sci. Technol. 2013, 48, 870–878. [Google Scholar] [CrossRef]
- Neacsu, N.A.; Madar, A. Artificial Sweeteners versus Natural Sweeteners. Bull. Transilv. Univ. Brasov 2014, 7, 59–64. [Google Scholar]
- Ramesh, M.; Muthuramas, A. Flavoring and Coloring Agents: Health Risk and Potential Problems. In Natural and artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–29. [Google Scholar]
- Damle, M.C.; Bhalekar, M.R.; Rao, S.; Godse, M. Formulation and Evaluation of Chewable Tablets of Pomegranate Peel Extract. J. Drug Deliv. Ther. 2019, 9, 318–321. [Google Scholar]
- Abdulmumeen, H.A.; Risikat, A.N.; Sururah, A.R. Food: Its Preservatives, Additives and Applications. Int. J. Chem. Biochm. Sci. 2012, 1, 36–47. [Google Scholar]
- Nishad, J.; Koley, T.K.; Varghese, E.; Kaur, C. Synergistic Effects of Nutmeg and Citrus Peel Extracts in Imparting Oxidative Stability in Meat Balls. Food Res. Int. 2018, 106, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Madhumita, R.; Ramalingam, C. Gum Acacia Coating with Garlic and Cinnamon as an Alternate, Natural Preservative for Meat and Fish. African J. Biotechnol. 2013, 12, 406–413. [Google Scholar] [CrossRef]
- Kordsardouei, H.; Barzegar, M.; Sahari, M.A. Application of Zataria Multiflora Boiss. and Cinnamon Zeylanicum Essential Oils as Two Natural Preservatives in Cake. Avicenna J. Phytomed. 2013, 3, 238–247. [Google Scholar] [CrossRef]
- Mugoyela, V.; Mugoyela, V.; Mwambete, K.D. Microbial Contamination of Nonsterile Pharmaceuticals in Public Hospital Settings. Ther. Clin. Risk Manag. 2010, 443. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, L. Microbial Contamination Control in the Pharmaceutical Industry; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Donald, J. Prevention of Microbial Contamination on Manufacturing. In Cosmetic Microbiology—A Practical Approach; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–71. [Google Scholar]
- Abourashed, E.A.; El-Alfy, A.T. Chemical Diversity and Pharmacological Significance of the Secondary Metabolites of Nutmeg (Myristica Fragrans Houtt). Phytochem. Rev. 2016, 15, 1035–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanghai-Vaijwade, D.N.; Kulkarni, S.R.; Sanghai, N.N. Nutmeg: A Promising Antibacterial Agent for Stability of Sweets. Int. J. Research Pharm. Chem. 2011, 1, 403–407. [Google Scholar]
- Takikawa, A.; Abe, K.; Yamamoto, M.; Ishimaru, S.; Yasui, M.; Okubo, Y.; Yokoigawa, K. Antimicrobial Activity of Nutmeg against Escherichia Coli O157. J. Biosci. Bioeng. 2002, 94, 315–320. [Google Scholar] [CrossRef]
- Firouzi, R.; Shekarforoush, S.S.; Nazer, A.H.; Borumand, Z.; Jooyandeh, A.R. Effects of Essential Oils of Oregano and Nutmeg on Growth and Survival of Yersinia Enterocolitica and Listeria Monocytogenes in Barbecued Chicken. J. Food Prot. 2007, 70, 2626–2630. [Google Scholar] [CrossRef]
- Matulyte, I.; Jekabsone, A.; Jankauskaite, L.; Zavistanaviciute, P.; Sakiene, V.; Bartkiene, E.; Ruzauskas, M.; Kopustinskiene, D.M.; Santini, A.; Bernatoniene, J. The Essential Oil and Hydrolats from Myristica Fragrans Seeds with Magnesium Aluminometasilicate. Foods 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, E.Y.; Lepesqueur, L.S.S.; Yassuda, C.G.; Samaranayake, L.P.; Parahitiyawa, N.B.; Balducci, I.; Koga-Ito, C.Y. Enterococcus Species in the Oral Cavity: Prevalence, Virulence Factors and Antimicrobial Susceptibility. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, E.T.; Mayer, M.P.A. Enterococcus Faecalis in Oral Infections. JBR J. Interdiscip. Med. Dent. Sci. 2014, 3, 1–5. [Google Scholar]
- Embuscado, M.E. Spices and Herbs: Natural Sources of Antioxidants—A Mini Review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Matulyte, I.; Marksa, M.; Ivanauskas, L.; Kalvenien e, Z.; Lazauskas, R.; Bernatoniene, J. GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica Fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules 2019, 24, 1062. [Google Scholar] [CrossRef] [Green Version]
- European Pharmacopoeia. Microbiological Examination of Non-Sterile Products: Test for Specified Micro-Organisms. In Europoean Pharmacopoeia 10.3; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 167–171. [Google Scholar]
- European Pharmacopoeia. Microbiological Quality of Non-Sterile Pharmaceutical Preparations Ans Substances for Pharmaceutical Use. In Europoean Pharmacopoeia 10.3; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 579–580. [Google Scholar]
- Morten, J.D.; Magnus, H.N.; Kurt, D.I. Soft, Chewable Gelatin-Based Pharmaceutical Oral Formulations: A Technical Approach. Pharm. Dev. Technol. 2018, 23, 504–511. [Google Scholar] [CrossRef]
- Pizzoni, D.; Compagnone, D.; Di Natale, C.; D’Alessandro, N.; Pittia, P. Evaluation of Aroma Release of Gummy Candies Added with Strawberry Flavours by Gas-Chromatography/Mass-Spectrometry and Gas Sensors Arrays. J. Food Eng. 2015, 167, 77–86. [Google Scholar] [CrossRef]
- Déléris, I.; Saint-Eve, A.; Dakowski, F.; Sémon, E.; Le Quéré, J.L.; Guillemin, H.; Souchon, I. The Dynamics of Aroma Release during Consumption of Candies of Different Structures, and Relationship with Temporal Perception. Food Chem. 2011, 127, 1615–1624. [Google Scholar] [CrossRef]
- Farahni, A.; Ansari, S.; Majzobi, M. Effect of Glycerol and Glucose Syrup on Sugar Crystallization in Figs. J. Sci. Technol. Agric. Nat. Resour. Source 2009, 1. [Google Scholar]
- Ballmann, C.; Müeller, B.W. Stabilizing Effect of Cetostearyl Alcohol and Glycerylmonstearate as Co-Emulsifiers on Hydrocarbon-Free O/W Glyceride Creams. Pharm. Dev. Technol. 2008, 13, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E. Glycerol Use in Dairy Diets: A Systemic Review. Anim. Nutr. 2019, 5, 209–216. [Google Scholar] [CrossRef]
- Dauqan, E.M.A.; Abdullah, A. Medicinal and Functional Values of Thyme (Thymus Vulgaris L.) Herb. J. Appl. Biol. Biotechnol. 2017, 5, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Zekovic, Z.P.; Lepojevic, Z.D.; Miloševič, S.; Markov, S.L. Tablets with Thyme (Thymus Vulgaris L.) Extracts. Acta Period. Technol. 2002, 33, 159–165. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of Whey Protein Purity and Glycerol Content upon Physical Properties of Edible Films Manufactured Therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Engineering ToolBox, Glycerine-Boiling and Freezing Points. Available online: https://www.engineeringtoolbox.com/glycerine-boiling-freezing-points-d_1590.html (accessed on 3 February 2021).
- Lane, L.B. Freezing Points of Glycerol and Its Aqueous Solutions. Ind. Eng. Chem. 1925, 17, 924. [Google Scholar] [CrossRef]
- Ratajczak, M.; Kubicka, M.M.; Kamińska, D.; Sawicka, P.; Długaszewska, J. Microbiological Quality of Non-Sterile Pharmaceutical Products. Saudi Pharm. J. 2015, 23, 303–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, S.P.; Sati, N. Artificial Preservatives and Their Harmful Effects: Looking Toward Nature for Safer Alternatives. Int. J. Pharm. Sci. Res. IJPSR 2013, 4, 2496–2501. [Google Scholar] [CrossRef]
- Shaikh, S.M.; Doijad, R.C.; Shete, A.S.; Sankpal, P.S. A Review on: Preservatives Used in Pharmaceuticals and Impacts on Health. PharmaTutor 2016, 4, 25–34. [Google Scholar]
- Dwivedi, S.; Prajapati, P.; Vyas, N.; Malviya, S.; Kharia, A. A Review on Food Preservation: Methods, Harmful Effects and Better Alternatives. Asian J. Pharm. Pharmacol. 2017, 3, 193–199. [Google Scholar]
- Netramai, S.; Kijchavengkul, T.; Sompoo, P.; Kungnimit, W. The Effect of Intrinsic and Extrinsic Factors on Moisture Sorption Characteristics of Hard Candy. J. Food Process. Preserv. 2018, 42, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Barringer, S. Effect of Hydrocolloids, Sugar, and Citric Acid on Strawberry Volatiles in a Gummy Candy. J. Food Process. Preserv. 2018, 42, 1–8. [Google Scholar] [CrossRef]








| Ingredients | Samples Series | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0 | G1 | Sb | E | C | Ae | Ee | Eo | Eos | H | |
| Gelatin (g) | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Distilled water (mL) | 20.0 | 18.0 | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 | 18.0 | 17.0 | 17.0 |
| Glycerol (g) | - | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Thyme syrup (g) | 25.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| Sodium benzoate solution 5% (mL) | - | - | 1.0 | - | - | - | - | - | - | - |
| 96% ethanol (mL) | - | - | - | 1.0 | - | - | - | - | - | - |
| Citric acid 50% solution (mL) | - | - | - | - | 1.0 | - | - | - | - | - |
| Aqueous nutmeg extract (mL) | - | - | - | - | - | 1.0 | - | - | - | - |
| Ethanol nutmeg extract (mL) | - | - | - | - | - | - | 1.0 | - | - | - |
| Nutmeg essential oil (µL) | - | - | - | - | - | - | - | 10.0 | - | - |
| Ethanol nutmeg essential oil 10% solution (mL) | - | - | - | - | - | - | - | - | 1.0 | - |
| Nutmeg hydrolat (mL) | - | - | - | - | - | - | - | - | - | 1.0 |
| Micro-Organisms | Equipment and Devices (Scales, Texture Analyzer, Tweezers, Silicone Form and etc.) | Containers (Plastic Box) (the Plastic Squeezable Bag Was Not Explored; It Was a Single-Use Plastic Bag, Taken Straight from the Supplier Box (Non-Contaminated)) |
|---|---|---|
| TAMC | <1.0 × 101 | <1.0 × 101 |
| TYMC | <1.0 × 101 | <1.0 × 101 |
| Bile-tolerant gram-negative bacteria | not detected | not detected |
| Salmonella spp. | not detected | not detected |
| Escherichia coli | not detected | not detected |
| Staphylococcus aureus | not detected | not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulyte, I.; Mataraite, A.; Velziene, S.; Bernatoniene, J. The Effect of Myristica fragrans on Texture Properties and Shelf-Life of Innovative Chewable Gel Tablets. Pharmaceutics 2021, 13, 238. https://doi.org/10.3390/pharmaceutics13020238
Matulyte I, Mataraite A, Velziene S, Bernatoniene J. The Effect of Myristica fragrans on Texture Properties and Shelf-Life of Innovative Chewable Gel Tablets. Pharmaceutics. 2021; 13(2):238. https://doi.org/10.3390/pharmaceutics13020238
Chicago/Turabian StyleMatulyte, Inga, Akvile Mataraite, Saule Velziene, and Jurga Bernatoniene. 2021. "The Effect of Myristica fragrans on Texture Properties and Shelf-Life of Innovative Chewable Gel Tablets" Pharmaceutics 13, no. 2: 238. https://doi.org/10.3390/pharmaceutics13020238