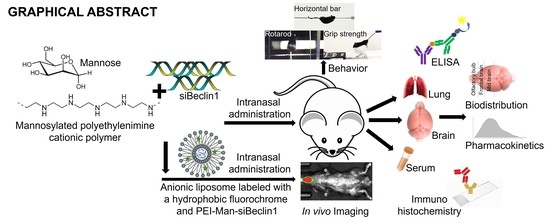
Targeting Beclin1 as an Adjunctive Therapy against HIV Using Mannosylated Polyethylenimine Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and HIV Infection
2.2. Transfection of siRNA into Microglia, Astrocytes, and Neurons
2.3. Immunochemistry
2.4. Time-Lapse Assessment of Neuronal Viability
2.5. Viability Assay
2.6. Animals
2.7. Intranasal Administration of siRNA-PEI Nanocomplex into C57BL/6 Mice
2.8. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.9. Nissl Staining
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Liposome Composition and In Vivo Imaging System (IVIS)
2.12. Statistical Analysis
3. Results
3.1. siBeclin1 Reduces Viral Production in HIV-Infected Human Microglia Co-Administered with Antiretroviral Drugs and Attenuates Secreted Viral-Induced Inflammatory Molecules in Human Microglia and Human Astrocytes
3.2. PEI-Man Targets Human Microglia and Human Astrocytes-Expressing Mannose Membrane Receptors and Does Not Exert Toxicity to Human Neuronal Cells
3.3. Biodistribution of the PEI-Man-siBeclin1 Nanoparticle in C57BL/6 Mice Brains after Intranasal Delivery
3.4. PEI-Man-siBeclin1 Nanoparticle Delivered to the Brain Causes Minimal Toxicity and Reduces the Secretion of Inflammatory Molecules
3.5. Biodistribution of DIR-Liposome-Nanoparticles in C57BL/6 Mice after Intranasal Delivery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Rizwanullah, M.; Amin, S.; Warsi, M.H.; Ahmad, M.Z.; Barkat, A. Nanostructured Lipid Carriers (NLCs): Nose-to-Brain Delivery and Theranostic Application. Curr. Drug Metab. 2020, 21, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Rajaram, M.V.S.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The Possible “Proton Sponge” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobbin, M.L.; Rossi, J.J. RNA Interference (RNAi)-Based Therapeutics: Delivering on the Promise? Annu. Rev. Pharmacol. Toxicol. 2016, 56, 103–122. [Google Scholar] [CrossRef]
- Boland, B.; Kumar, A.; Lee, S.; Platt, F.M.; Wegiel, J.; Yu, W.H.; Nixon, R.A. Autophagy Induction and Autophagosome Clearance in Neurons: Relationship to Autophagic Pathology in Alzheimer’s Disease. J. Neurosci. 2008, 28, 6926–6937. [Google Scholar] [CrossRef] [Green Version]
- Bortolozzi, A.; Castañé, A.; Semakova, J.; Santana, N.; Alvarado, G.; Cortés, R.; Ferrés-Coy, A.; Fernandez, G.; Carmona, M.C.; Toth, M.; et al. Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol. Psychiatry 2011, 17, 612–623. [Google Scholar] [CrossRef] [Green Version]
- Braschi, C.; Capsoni, S.; Narducci, R.; Poli, A.; Sansevero, G.; Brandi, R.; Maffei, L.; Cattaneo, A.; Berardi, N. Intranasal delivery of BDNF rescues memory deficits in AD11 mice and reduces brain microgliosis. Aging Clin. Exp. Res. 2020, 1–16. [Google Scholar] [CrossRef]
- Burudi, E.; Riese, S.; Stahl, P.D. Identification and functional characterization of the mannose receptor in astrocytes. Glia 1998, 25, 44–55. [Google Scholar] [CrossRef]
- Castanotto, D.; Rossi, J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009, 457, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zhang, X.; Jia, L.; Prud’Homme, R.K.; Szekely, Z.; Sinko, P.J. Optimal structural design of mannosylated nanocarriers for macrophage targeting. J. Control Release 2014, 194, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhou, Q.; Ni, Y.; Le, W. Autophagy and Alzheimer’s Disease. Adv. Exp. Med. Biol. 2020, 1207, 3–19. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creusat, G.; Rinaldi, A.-S.; Weiss, E.; Elbaghdadi, R.; Remy, J.-S.; Mulherkar, R.; Zuber, G. Proton Sponge Trick for pH-Sensitive Disassembly of Polyethylenimine-Based siRNA Delivery Systems. Bioconjugate Chem. 2010, 21, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Dinkins, C.; Pilli, M.; Kehrl, J.H. Roles of autophagy in HIV infection. Immunol. Cell Biol. 2014, 93, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, H.; Grotepas, C.B.; McMillan, J.M.; Destache, C.J.; Chaubal, M.; Werling, J.; Kipp, J.; Rabinow, B.; Gendelman, H.E. Macrophage Delivery of Nanoformulated Antiretroviral Drug to the Brain in a Murine Model of NeuroAIDS. J. Immunol. 2009, 183, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Ebato, C.; Uchida, T.; Arakawa, M.; Komatsu, M.; Ueno, T.; Komiya, K.; Azuma, K.; Hirose, T.; Tanaka, K.; Kominami, E.; et al. Autophagy Is Important in Islet Homeostasis and Compensatory Increase of Beta Cell Mass in Response to High-Fat Diet. Cell Metab. 2008, 8, 325–332. [Google Scholar] [CrossRef] [Green Version]
- El-Hage, N.; Rodriguez, M.; Dever, S.M.; Masvekar, R.R.; Gewirtz, D.A.; Shacka, J.J. HIV-1 and Morphine Regulation of Autophagy in Microglia: Limited Interactions in the Context of HIV-1 Infection and Opioid Abuse. J. Virol. 2014, 89, 1024–1035. [Google Scholar] [CrossRef] [Green Version]
- Ene, L.; Duiculescu, D.; Ruta, S.M. How much do antiretroviral drugs penetrate into the central nervous system? J. Med. Life 2011, 4, 432–439. [Google Scholar]
- Espert, L.; Varbanov, M.; Robert-Hebmann, V.; Sagnier, S.; Robbins, I.; Sanchez, F.; Lafont, V.; Biard-Piechaczyk, M. Differential Role of Autophagy in CD4 T Cells and Macrophages during X4 and R5 HIV-1 Infection. PLoS ONE 2009, 4, e5787. [Google Scholar] [CrossRef] [PubMed]
- Falcone, J.A.; Salameh, T.S.; Yi, X.; Cordy, B.J.; Mortell, W.G.; Kabanov, A.V.; Banks, W.A. Intranasal Administration as a Route for Drug Delivery to the Brain: Evidence for a Unique Pathway for Albumin. J. Pharmacol. Exp. Ther. 2014, 351, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Fields, J.; Dumaop, W.; Elueteri, S.; Campos, S.; Serger, E.; Trejo, M.; Kosberg, K.; Adame, A.; Spencer, B.; Rockenstein, E.; et al. HIV-1 Tat Alters Neuronal Autophagy by Modulating Autophagosome Fusion to the Lysosome: Implications for HIV-Associated Neurocognitive Disorders. J. Neurosci. 2015, 35, 1921–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Weng, Y.; Xia, X.; Liang, X.; Huang, Y. Clinical advances of siRNA therapeutics. J. Gene Med. 2019, 21, e3097. [Google Scholar] [CrossRef]
- Irache, J.; Juan, M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin. Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Chakraborty, A.; Lee, J.-H.; Knowles, J.C.; Kim, H.-W. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Juhász, G.; Érdi, B.; Sass, M.; Neufeld, T.P. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007, 21, 3061–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaul, M.; Garden, G.A.; Lipton, S.A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001, 410, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N.; et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep. 2016, 6, 25309. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-D.; Shin, J.-H.; Kim, S.-W.; Choi, S.; Ahn, J.; Han, P.-L.; Park, J.-S.; Lee, J.-K. Intranasal Delivery of HMGB1 siRNA Confers Target Gene Knockdown and Robust Neuroprotection in the Postischemic Brain. Mol. Ther. 2012, 20, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Kranick, S.M.; Nath, A. Neurologic Complications of HIV-1 Infection and Its Treatment in the Era of Antiretroviral Therapy. Continuum 2012, 18, 1319–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, V.V.; Maday, S. Compartment-specific dynamics and functions of autophagy in neurons. Dev. Neurobiol. 2018, 78, 298–310. [Google Scholar] [CrossRef]
- Kumar, R.; Gulati, M.; Singh, S.K.; Sharma, D.; Porwal, O. Road From Nose to Brain for Treatment of Alzheimer: The Bumps and Humps. CNS Neurol. Disord. Drug Targets 2020, 19, 663–675. [Google Scholar] [CrossRef]
- Lahiri, C.D.; Reed-Walker, K.; Sheth, A.N.; Acosta, E.P.; Vunnava, A.; Ofotokun, I. Cerebrospinal fluid concentrations of tenofovir and emtricitabine in the setting of HIV-1 protease inhibitor-based regimens. J. Clin. Pharmacol. 2015, 56, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Lamers, S.L.; Salemi, M.; Galligan, D.C.; Morris, A.; Gray, R.; Fogel, G.; Zhao, L.; McGrath, M.S. Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. J. NeuroVirology 2010, 16, 230–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapierre, J.; Rodriguez, M.; Ojha, C.R.; El-Hage, N. Critical Role of Beclin1 in HIV Tat and Morphine-Induced Inflammation and Calcium Release in Glial Cells from Autophagy Deficient Mouse. J. Neuroimmune Pharmacol. 2018, 13, 355–370. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Li, K.; Deng, L.; Wang, H. Combination of an Autophagy Inducer and an Autophagy Inhibitor: A Smarter Strategy Emerging in Cancer Therapy. Front. Pharmacol. 2020, 11, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-F. Clinical trials of intranasal delivery for treating neurological disorders—A critical review. Expert Opin. Drug Deliv. 2011, 8, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Kim, B.O.; Gattone, V.H.; Li, J.; Nath, A.; Blum, J.; He, J.J. CD4-Independent Infection of Astrocytes by Human Immunodeficiency Virus Type 1: Requirement for the Human Mannose Receptor. J. Virol. 2004, 78, 4120–4133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, S.D.; Law, W.-C.; Aalinkeel, R.; Reynolds, J.L.; Nair, B.B.; Sykes, D.E.; Yong, K.-T.; Roy, I.; Prasad, P.N.; Schwartz, S.A. Anti-HIV-1 nanotherapeutics: Promises and challenges for the future. Int. J. Nanomed. 2012, 7, 5301–5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammana, S.; Fagone, P.; Cavalli, E.; Basile, M.S.; Petralia, M.C.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The Role of Macrophages in Neuroinflammatory and Neurodegenerative Pathways of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis: Pathogenetic Cellular Effectors and Potential Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.W.; Shao, Q.; Vig, P.J.; Bidwell, G.L. Intranasal administration of elastin-like polypeptide for therapeutic delivery to the central nervous system. Drug Des. Dev. Ther. 2016, 10, 2803–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minami, S.S.; Sun, B.; Popat, K.C.; Kauppinen, T.M.; A Pleiss, M.; Zhou, Y.; Ward, M.E.; Floreancig, P.E.; Mucke, L.; Desai, T.A.; et al. Selective targeting of microglia by quantum dots. J. Neuroinflammation 2012, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Navone, F.; Genevini, P.; Borgese, N. Autophagy and Neurodegeneration: Insights from a Cultured Cell Model of ALS. Cells 2015, 4, 354–386. [Google Scholar] [CrossRef]
- Nemchenko, A.; Chiong, M.; Turer, A.; Lavandero, S.; Hill, J.A. Autophagy as a therapeutic target in cardiovascular disease. J. Mol. Cell. Cardiol. 2011, 51, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolting, T.; Lindecke, A.; Koutsilieri, E.; Maschke, M.; Husstedt, I.-W.; Sopper, S.; Stuve, O.; Hartung, H.-P.; Arendt, G. Measurement of soluble inflammatory mediators in cerebrospinal fluid of human immunodeficiency virus–positive patients at distinct stages of infection by solid-phase protein array. J. NeuroVirology 2009, 15, 390–400. [Google Scholar] [CrossRef]
- Nowacek, A.; Gendelman, H.E. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine 2009, 4, 557–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.; Maldarelli, F.; Wiegand, A.; Bernstein, B.; Hanna, G.J.; Brun, S.C.; Kempf, D.J.; Mellors, J.W.; Coffin, J.M.; King, M.S. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2008, 105, 3879–3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathan, S.A.; Iqbal, Z.; Zaidi, S.M.; Talegaonkar, S.; Vohra, D.; Jain, G.K.; Azeem, A.; Jain, N.; Lalani, J.R.; Khar, R.K.; et al. CNS Drug Delivery Systems: Novel Approaches. Recent Patents Drug Deliv. Formul. 2009, 3, 71–89. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renner, D.B.; Frey, W.H.; Hanson, L.R. Intranasal delivery of siRNA to the olfactory bulbs of mice via the olfactory nerve pathway. Neurosci. Lett. 2012, 513, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Lapierre, J.; Ojha, C.R.; Estrada-Bueno, H.; Dever, S.M.; Gewirtz, D.A.; Kashanchi, F.; El-Hage, N. Importance of Autophagy in Mediating Human Immunodeficiency Virus (HIV) and Morphine-Induced Metabolic Dysfunction and Inflammation in Human Astrocytes. Viruses 2017, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Lapierre, J.; Ojha, C.R.; Kaushik, A.; Batrakova, E.; Kashanchi, F.; Dever, S.M.; Nair, M.; El-Hage, N. Intranasal drug delivery of small interfering RNA targeting Beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef]
- Régnier-Vigouroux, A. The Mannose Receptor in the Brain. Int. Rev. Cytol. 2003, 226, 321–342. [Google Scholar] [CrossRef]
- Saribas, A.S.; Khalili, K.; Sariyer, I.K. Dysregulation of autophagy by HIV-1 Nef in human astrocytes. Cell Cycle 2015, 14, 2899–2904. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Kern, W.; Giese, R.; Hallschmid, M.; Enders, A. Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome: An exploratory clinical trial. J. Med. Genet. 2008, 46, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, J.; Höbel, S.; Bakowsky, U.; Aigner, A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials 2010, 31, 6892–6900. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Shibata, M.; Lu, T.; Furuya, T.; Degterev, A.; Mizushima, N.; Yoshimori, T.; Macdonald, M.; Yankner, B.; Yuan, J. Regulation of Intracellular Accumulation of Mutant Huntingtin by Beclin 1. J. Biol. Chem. 2006, 281, 14474–14485. [Google Scholar] [CrossRef] [Green Version]
- Sukegawa, S.; Miyagi, E.; Bouamr, F.; Farkašová, H.; Strebel, K. Mannose Receptor 1 Restricts HIV Particle Release from Infected Macrophages. Cell Rep. 2018, 22, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.B.; Gnanadhas, D.P.; Dash, P.K.; Machhi, J.; Lin, Z.; McMillan, J.; Edagwa, B.; Gelbard, H.; Gendelman, H.E.; Gorantla, S. Modulating cellular autophagy for controlled antiretroviral drug release. Nanomedicine 2018, 13, 2139–2154. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, H.M.; Ducommun, M.M.; Briley, W.E.; Shea, L.D. Gene delivery to overcome astrocyte inhibition of axonal growth: An in vitro Model of the glial scar. Biotechnol. Bioeng. 2013, 110, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varatharajan, L.; Thomas, S.A. The transport of anti-HIV drugs across blood–CNS interfaces: Summary of current knowledge and recommendations for further research. Antivir. Res. 2009, 82, A99–A109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgin, H.W.; Levine, B. Autophagy genes in immunity. Nat. Immunol. 2009, 10, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Winslow, A.R.; Rubinsztein, D.C. Autophagy in neurodegeneration and development. Biochim. Biophys. Acta 2008, 1782, 723–729. [Google Scholar] [CrossRef]
- Wong, H.L.; Chattopadhyay, N.; Wu, X.Y.; Bendayan, R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv. Drug Deliv. Rev. 2010, 62, 503–517. [Google Scholar] [CrossRef]
- Yang, J.-P.; Liu, H.-J.; Cheng, S.; Wang, Z.-L.; Cheng, X.; Yu, H.-X.; Liu, X.-F. Direct transport of VEGF from the nasal cavity to brain. Neurosci. Lett. 2009, 449, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The Role of Autophagy in Cancer: Therapeutic Implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, T.; Sajid, A.; Mathew, B.; Uddin, S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2019, 27, 19151–19168. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Zade, A.K.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Ho, P.Y.; Tu, M.-J.; Jilek, J.L.; Chen, Q.-X.; Zeng, S.; Yu, A.-M. Lipidation of polyethylenimine-based polyplex increases serum stability of bioengineered RNAi agents and offers more consistent tumoral gene knockdown in vivo. Int. J. Pharm. 2018, 547, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Masliah, E.; Spector, S.A. Autophagy Is Increased in Postmortem Brains of Persons With HIV-1-Associated Encephalitis. J. Infect. Dis. 2011, 203, 1647–1657. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, M.; Soler, Y.; Muthu Karuppan, M.K.; Zhao, Y.; Batrakova, E.V.; El-Hage, N. Targeting Beclin1 as an Adjunctive Therapy against HIV Using Mannosylated Polyethylenimine Nanoparticles. Pharmaceutics 2021, 13, 223. https://doi.org/10.3390/pharmaceutics13020223
Rodriguez M, Soler Y, Muthu Karuppan MK, Zhao Y, Batrakova EV, El-Hage N. Targeting Beclin1 as an Adjunctive Therapy against HIV Using Mannosylated Polyethylenimine Nanoparticles. Pharmaceutics. 2021; 13(2):223. https://doi.org/10.3390/pharmaceutics13020223
Chicago/Turabian StyleRodriguez, Myosotys, Yemmy Soler, Mohan Kumar Muthu Karuppan, Yuling Zhao, Elena V. Batrakova, and Nazira El-Hage. 2021. "Targeting Beclin1 as an Adjunctive Therapy against HIV Using Mannosylated Polyethylenimine Nanoparticles" Pharmaceutics 13, no. 2: 223. https://doi.org/10.3390/pharmaceutics13020223