Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review
Abstract
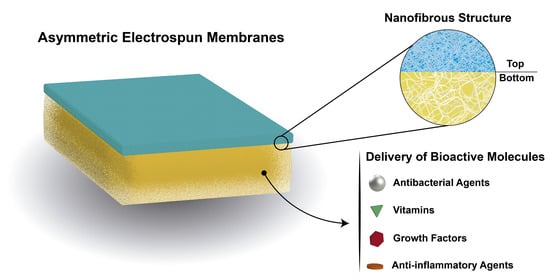
:1. Introduction
2. Asymmetric Membranes
2.1. Electrospinning Technique
2.2. Asymmetric Electrospun Membranes
3. Electrospun Asymmetric Membranes as Delivery Systems of Biomolecules
3.1. Electrospun Asymmetric Membranes with Antibacterial Activity
3.2. Electrospun Asymmetric Membranes Loaded with Bioactive Molecules that Improve the Healing Process
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra, A.; Belinha, J.; Jorge, R.N. Modelling skin wound healing angiogenesis: A review. J. Theor. Biol. 2018, 459, 1–17. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K.F. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.F.; Bartolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, S.; Baganizi, D.R.; Sahu, R.; Dosunmu, E.; A Chaudhari, A.; Vig, K.; Pillai, S.R.; Singh, S.R.; Dennis, V. Immunological challenges associated with artificial skin grafts: Available solutions and stem cells in future design of synthetic skin. J. Biol. Eng. 2017, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin substitutes and bioscaffolds: Temporary and permanent coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging innovative wound dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- In, S.M.; An, H.G.; Kim, J.-Y.; Lee, K.-I. Columellar Wound Immediately After Open Rhinoseptoplasty Treated With Application of DuoDERM Extra Thin. J. Craniofacial Surg. 2020, 32, e98. [Google Scholar] [CrossRef]
- Alinejad, F.; Momeni, M.; Fatemi, M.J.; Dahmardehei, M.; Naderi, S.; Akhoondinasab, M.R.; Zayedly, M.; Mahboubi, O.; Rahbar, H. Comparing the effect of two types of silver nano-crystalline dressings (acticoat and agcoat) in the treatment of full thickness burn wound. Iran. J. Microbiol. 2018, 10, 378. [Google Scholar]
- Kuo, F.-C.; Chen, B.; Lee, M.S.; Yen, S.-H.; Wang, J.-W. AQUACEL® Ag surgical dressing reduces surgical site infection and improves patient satisfaction in minimally invasive total knee arthroplasty: A prospective, randomized, controlled study. Biomed Res. Int. 2017, 2017, 1262108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Huan, Z.; Zhang, L.; Chang, J. The clinical application of a silicate-based wound dressing (DermFactor®) for wound healing after anal surgery: A randomized study. Int. J. Surg. 2018, 52, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Makin, I.; Skiba, J.; Ho, A.; Housler, G.; Stojadinovic, A.; Izadjoo, M. Antibacterial efficacy testing of a bioelectric wound dressing against clinical wound pathogens. Open Microbiol. J. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. European Journal of Pharmaceutics and Biopharmaceutics Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Dias, J.; Granja, P.; Bártolo, P. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Goodarzi, P.; Falahzadeh, K.; Nematizadeh, M.; Farazandeh, P.; Payab, M.; Larijani, B.; Beik, A.T.; Arjmand, B. Tissue engineered skin substitutes. In Cell Biology and Translational Medicine; Springer: Berlin/Heidelberg, Germany, 2018; Volume 3, pp. 143–188. [Google Scholar]
- Pan, C. Gas separation by permeators with high-flux asymmetric membranes. Aiche J. 1983, 29, 545–552. [Google Scholar] [CrossRef]
- Opong, W.S.; Zydney, A.L. Diffusive and convective protein transport through asymmetric membranes. Aiche J. 1991, 37, 1497–1510. [Google Scholar] [CrossRef]
- Zhang, D.; Patel, P.; Strauss, D.; Qian, X.; Wickramasinghe, S.R. Modeling tangential flow filtration using reverse asymmetric membranes for bioreactor harvesting. Biotechnol. Prog. 2020, e3084. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electrospun scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly (vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membr. Sci. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Baker, A.B. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef]
- Franco, R.A.; Min, Y.-K.; Yang, H.-M.; Lee, B.-T. Fabrication and biocompatibility of novel bilayer scaffold for skin tissue engineering applications. J. Biomater. Appl. 2013, 27, 605–615. [Google Scholar] [CrossRef]
- Figueira, D.R.; Miguel, S.P.; De Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone-hyaluronic acid/chitosan-zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Membr. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Hinrichs, W.; Lommen, E.J.C.M.P.; Wildevuur, C.R.H.; Feijen, J. Fabrication and characterization of an asymmetric polyurethane membrane for use as a wound dressing. J. Appl. Biomater. 1992, 3, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A.; Omidifar, N.; Shokripour, M. Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications. Symmetry 2020, 12, 1100. [Google Scholar] [CrossRef]
- Marcano, A.; Haidar, N.B.; MaraisOrcid, S.; Valleton, J.-M.; Duncan, A.C. Designing biodegradable PHA-based 3D scaffolds with antibiofilm properties for wound dressings: Optimization of the microstructure/nanostructure. Acs Biomater. Sci. Eng. 2017, 3, 3654–3661. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.C.; Kosar, W.; Zhang, Y.; Feng, X. A study of thermodynamics and kinetics pertinent to formation of PVDF membranes by phase inversion. Desalination 2013, 309, 156–164. [Google Scholar] [CrossRef]
- Mi, F.-L.; Wu, Y.-B.; Shyu, S.-S.; Chao, A.-C.; Lai, J.-Y.; Su, C.-C. Asymmetric chitosan membranes prepared by dry/wet phase separation: A new type of wound dressing for controlled antibacterial release. J. Membr. Sci. 2003, 212, 237–254. [Google Scholar] [CrossRef]
- Cardea, S.; Sessa, M.; Reverchon, E. Supercritical phase inversion to form drug-loaded poly (vinylidene fluoride-co-hexafluoropropylene) membranes. Ind. Eng. Chem. Res. 2010, 49, 2783–2789. [Google Scholar] [CrossRef]
- Temtem, M.; Casimiro, T.; Aguiar-Ricardo, A. Solvent power and depressurization rate effects in the formation of polysulfone membranes with CO2-assisted phase inversion method. J. Membr. Sci. 2006, 283, 244–252. [Google Scholar] [CrossRef]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Park, S.; Park, K.; Yoon, H.; Son, J.; Min, T.; Kim, G. Apparatus for preparing electrospun nanofibers: Designing an electrospinning process for nanofiber fabrication. Polym. Int. 2007, 56, 1361–1366. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunne, N.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N. Cell electrospinning: A novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Hu, Q. A novel bioactive membrane by cell electrospinning. Exp. Cell Res. 2015, 338, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gilbert-Honick, J.; Somers, S.M.; Mao, H.-Q.; Grayson, W. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles. Biochem. Biophys. Res. Commun. 2019, 516, 558–564. [Google Scholar] [CrossRef]
- Ang, H.Y.; Irvine, S.A.; Avrahami, R.; Sarig, U.; Bronshtein, T.; Zussman, E.; Boey, F.Y.C.; Machluf, M.; Venkatraman, S.S. Characterization of a bioactive fiber scaffold with entrapped HUVECs in coaxial electrospun core-shell fiber. Biomatter 2014, 4, e28238. [Google Scholar] [CrossRef] [Green Version]
- De Prá, M.A.A.; Ribeiro-do-Valle, R.M.; Maraschin, M.; Veleirinho, B. Effect of collector design on the morphological properties of polycaprolactone electrospun fibers. Mater. Lett. 2017, 193, 154–157. [Google Scholar] [CrossRef]
- Sequeira, R.S.; Miguel, S.P.; Cabral, C.S.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Development of a poly (vinyl alcohol)/lysine electrospun membrane-based drug delivery system for improved skin regeneration. Int. J. Pharm. 2019, 570, 118640. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Lin, Z.Y.; Wong, K.K.Y.; Lin, M.; Yildirimer, L.; Zhao, X. Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov. Today 2017, 22, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Reshmi, C.; Menon, T.; Binoy, A.; Mishra, N.; Elyas, K.K.; Sujith, A. Poly (L-lactide-co-caprolactone)/collagen electrospun mat: Potential for wound dressing and controlled drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 645–657. [Google Scholar] [CrossRef]
- Yu, B.; He, C.; Wang, W.; Ren, Y.; Yang, J.; Guo, S.; Zheng, Y.; Shi, X. Asymmetric Wettable Composite Wound Dressing Prepared by Electrospinning with Bioinspired Micropatterning Enhances Diabetic Wound Healing. ACS Appl. Bio Mater. 2020, 3, 5383–5394. [Google Scholar] [CrossRef]
- Alves, P.; Santos, M.; Mendes, S.; Miguel, S.P.; De Sá, K.D.; Cabral, C.S.D.; Correia, I.J.; Ferreira, P. Photocrosslinkable nanofibrous asymmetric membrane designed for wound dressing. Polymers 2019, 11, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, E.; Maisuria, V.; Tufenkji, N.; Cerruti, M. Antibacterial Properties of PLGA Electrospun Scaffolds Containing Ciprofloxacin Incorporated by Blending or Physisorption. Acs Appl. Bio Mater. 2018, 1, 627–635. [Google Scholar] [CrossRef]
- Zahid, S.; Khalid, H.; Ikram, F.; Iqbal, H.; Samie, M.; Shahzadi, L.; Shah, A.T.; Yar, M.; Chaudhry, A.A.; Awan, S.J.; et al. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater. Sci. Eng. C 2019, 101, 438–447. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2014, 28, 843–863. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B 2016, 4, 4801–4812. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, T.; Xin, Y.; Zhang, Z.; Ren, Z.; Lei, J.; Chu, B.; Wang, Y.; Tang, S. Nanofibrous asymmetric membranes self-organized from chemically heterogeneous electrospun mats for skin tissue engineering. Biomed. Mater. 2016, 11, 035019. [Google Scholar] [CrossRef]
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Gorain, B. Delivery of therapeutics from layer-by-layer electrospun nanofiber matrix for wound healing: An update. J. Pharm. Sci. 2020, 110, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, Y.; Lee, K.; Lai, J.-Y. A three-dimensional dual-layer nano/microfibrous structure of electrospun chitosan/poly (d, l-lactide) membrane for the improvement of cytocompatibility. J. Membr. Sci. 2014, 450, 224–234. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Castellano, M.; Vicini, S. Multilayer Alginate–Polycaprolactone Electrospun Membranes as Skin Wound Patches with Drug Delivery Abilities. ACS Appl. Mater. Interfaces 2020, 12, 31162–31171. [Google Scholar] [CrossRef] [PubMed]
- Aragón, J.; Costa, C.; Coelhoso, I.; Mendoza, G.; Aguiar-Ricardo, A.; Irusta, S. Electrospun asymmetric membranes for wound dressing applications. Mater. Sci. Eng. C 2019, 103, 109822. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun polycaprolactone/aloe vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Yang, S.; Liu, P.; Zhang, B. Electrospun PCL/mupirocin and chitosan/lidocaine hydrochloride multifunctional double layer nanofibrous scaffolds for wound dressing applications. Int. J. Nanomed. 2018, 13, 5287. [Google Scholar] [CrossRef] [Green Version]
- Rezk, A.I.; Lee, J.Y.; Son, B.C.; Park, C.H.; Kim, C.S. Bi-layered Nanofibers Membrane Loaded with Titanium Oxide and Tetracycline as Controlled Drug Delivery System for Wound Dressing Applications. Polymers 2019, 11, 1602. [Google Scholar] [CrossRef] [Green Version]
- Amini, F.; Semnani, D.; Karbasi, S.; Banitaba, S.N. A novel bilayer drug-loaded wound dressing of PVDF and PHB/Chitosan nanofibers applicable for post-surgical ulcers. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 772–777. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Tong, Y.; Jiang, Z.; Wang, C. Nitrofurazone-loaded electrospun PLLA/sericin-based dual-layer fiber mats for wound dressing applications. RSC Adv. 2015, 5, 16940–16949. [Google Scholar] [CrossRef]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the design and development of “fast-dissolving” electrospun nanofibers based drug delivery systems-A systematic review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C 2018, 90, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.; Sadarani, B.; Majumdar, A.; Bhullar, S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 2017, 41, 124–133. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, S.; Liu, Z.; Yang, W.; Lin, Y.; Qian, H.; Gao, F.; Li, G. Investigation on the structural properties of GaN films grown on La0. 3Sr1. 7AlTaO6 substrates. Mater. Res. Express 2014, 1, 025903. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, G.; Zhou, G.; Yi, W.; Zheng, X.; Zhou, S. Thermally switched release from a Nanogel-in-microfiber device. Adv. Healthc. Mater. 2015, 4, 1658–1663. [Google Scholar] [CrossRef]
- Yohe, S.T.; Kopechek, J.A.; Porter, T.M.; Colson, Y.L.; Grinstaff, M. Triggered drug release from Superhydrophobic meshes using high-intensity focused ultrasound. Adv. Healthc. Mater. 2013, 2, 1204–1208. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Song, L.; Fan, Y.; Tian, L.; Luan, S.; Niu, S.; Ren, L.; Ming, W.; Zhao, J. Synergistic photodynamic and photothermal antibacterial nanocomposite membrane triggered by single NIR light source. ACS Appl. Mater. Interfaces 2019, 11, 26581–26589. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Aavani, F.; Khorshidi, S.; Karkhaneh, A. A concise review on drug-loaded electrospun nanofibres as promising wound dressings. J. Med. Eng. Technol. 2019, 43, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Controlled release of multiple epidermal induction factors through core–shell nanofibers for skin regeneration. Eur. J. Pharm. Biopharm. 2013, 85, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Palmieri, B.; Vadalà, M.; Laurino, C. Nutrition in wound healing: Investigation of the molecular mechanisms, a narrative review. J. Wound Care 2019, 28, 683–693. [Google Scholar] [CrossRef]
- Ranjith, R.; Balraj, S.; Ganesh, J.; Milton, M.J. Therapeutic agents loaded chitosan-based nanofibrous mats as potential wound dressings: A review. Mater. Today Chem. 2019, 12, 386–395. [Google Scholar] [CrossRef]






| Main Polymer in Top Layer | Layer | Composition of the Layers | Fibers Average Diameter (nm) | Porosity (%) | Water Contact Angle | WVTR (g × m−2 × day−1) | Swelling Capacity | Degradation Profile | Ref |
|---|---|---|---|---|---|---|---|---|---|
| PCL | Top layer | PCL; PLA | 691 ± 282 | N.A. | 126.8 ± 5.7° | N.A. | N.A. | 1.54 ± 0.22%, PBS, pH 7.4, 3 days | [55] |
| Bottom layer | GelMA; ChMA | 477 ± 228 | 88.2 ± 6.1° to 46.7 ± 2.2° after 20 s | 5.63 ± 0.21%, PBS, pH 7.4, 3 days | |||||
| Top layer | CS; PCL | 370 ± 264 | ≈96.1 | 82.4 ± 6.4° | ≈ 2536 | ≈135.2%, PBS, pH 7.4, 4 h | 30%, PBS, pH 5.5, 15 days ≈4.8%, PBS, pH 7.4, 15 days | [26] | |
| Bottom layer | HA; PEO | 136 ± 61 | |||||||
| Top layer | PCL | 300 ± 50 | N.A. | 160° | N.A. | N.A. | N.A. | [65] | |
| Bottom layer | SAG; ZnO nanoparticles | 100 ± 30 | 30° | ||||||
| Top layer | PCL | 360 ± 68 | N.A. | Sample I: 135° Sample II: 120° to 119° after 20 s | Sample I: 2280 ± 153–2992 ± 72 Sample II: 2506 ± 42–4552 ± 82 | Sample I: 400% in TRIS, pH 8, 2 h; after 24 h, medium changed to AES, pH 5, swelling decreased to 250% Sample II: 350%, TRIS, pH 8, 2 h; after 24 h, medium changed to AES, pH 5, swelling decreased to 300% | Sample I: ≈2.9% in TRIS, pH 8; after 1 week, medium changed to PBS, pH 7.4, weight loss increased to 3.5%; after another 1 week, medium changed to AES, pH 5, weight loss increased to ≈4.2% Sample II: ≈4.0% in TRIS, pH 8; after 1 week medium changed to PBS, pH 7.4, weight loss decreased to ≈3.6%; after another 1 week, medium changed to AES, pH 5, weight loss increased to ≈4.6% | [66] | |
| Bottom layer | PVAc Sample I-PVAc in DMF/ETOH; Sample II-PVAc in DMF) | Sample I: 600 ± 100 Sample II: 3000 ± 1000 | Sample: 120° to 111° after 20 s Sample II: 76° to 27° after 20 s | ||||||
| Top layer | HA; PCL | 472 ± 192 | 90.40 ± 4.25 | 120.20 ± 0.85° | 1762.91 ± 187.50 | N.A. | ≈10%, PBS, pH 5.5, 7 days | [28] | |
| Bottom layer | CS; ZN; SA | 530 ± 180 | 101.96 ± 5.08° | ||||||
| Top layer | PCL | 385 ± 134 | 55 ± 5 | 126.2 ± 1.21° | 1252.35 ± 21.22 | Ratio ≈20, PBS, pH 5, 30 days | ≈30%, PBS containing lysozyme, 30 days | [67] | |
| Bottom layer | CS; AV; PEO | 152 ± 54 | 97.8 ± 4.5 | 69.06 ± 3.78° | |||||
| Top layer | SF; PCL | 615.9 ± 190.4 | 74.78 ± 6.98 | 103.10 ± 6.57° | 2070.62 ± 102.52 | Ratio ≈42, PBS, pH 8, Ratio ≈39.4, PBS, pH 5 | 23%, PBS, pH 7.4, 7 days | [20] | |
| Bottom layer | SF; HA; THY | 412.7 ± 106.7 | 38.77 ± 5.32° | ||||||
| Top layer | PCL; mupirocin | 1031 ± 227 | ≈ 78.2 | 34.6 ± 4.6° | N.A. | 622%, PBS, pH 7.4, 24 h | N.A. | [68] | |
| Bottom layer | CS; LID | 735 ± 152 | |||||||
| PeCL | Top layer | PeCL | 539 | N.A. | 117° | N.A. | N.A. | N.A. | [69] |
| Bottom layer | PDO; TiO2 nanoparticles (concentration of 3% (PP3T5T) and 5% (PP5T5T)); TTC | PP3T5T: 611 PP5T5T: 679 | PP3T5T - 48° PP5T5T - 79° | ||||||
| Top layer | PeCL (nylon mesh pore size 40 (PeCL40) or 80 μm (PeCL80)) | 270 | N.A. | PeCL40: 145.24 ± 3.10° PeCL80: 138.88 ± 3.64° | 1556.66 ± 37.79 | N.A. | N.A. | [54] | |
| Bottom layer | Gel | 144.9 ± 56.92 | 39.93 ± 3.03° | ||||||
| PLA | Top layer | PLA; VE | 2150 | N.A. | N.A. | N.A. | ≈11.82%, PBS, pH 7–7.6, 24 h | ≈5.7% in PBS, ≈8.2%, in lysozyme solution, and ≈1.5% in H2O2, after 21 days | [57] |
| Bottom layer | PCL; VE | 6094 | |||||||
| BGB | Top layer | BGB | 410 ± 186 | N.A. | 126.4° | N.A. | ≈50%, deionized water, 24 h | N.A. | [61] |
| Bottom layer | BGA | 150 ± 58 | 86 to 0° after 10 s | ≈400%, deionized water, 24 h | |||||
| PVDF | Top layer | PVDF | 207 | N.A. | N.A. | N.A. | Sample I: ≈34.62%, sample II: ≈41.92%, and sample III: ≈48.85%, in PBS, pH 7.4, 336 h | Sample I: ≈31.53%, sample II: ≈39.29%, and sample III: ≈41.18%, in PBS, pH 7.4, 336 h | [70] |
| Bottom layer | PHB; CS Sample I: PHB90%-CS10%; sample II: PHB85%-CS15%; sample III: PHB80%-CS20% | 100 | |||||||
| PLLA | Top layer | PLLA; SS (1:1, 2:1, 4:1); NFZ (0.2%, 0.5%, 1.0%) | 413 to 1095 | 75.14 ± 5.43 to 78.35 ± 2.38 | ≈60.0 to ≈143.3° | 3161.45 ± 64.97 to 3289.40 ± 58.11 | N.A. | N.A. | [71] |
| Bottom layer | NFZ; PLLA | 814.36 ± 9.93 | 125.7° |
| Composition | Antibacterial Activity | Antioxidant Activity | Cell Behavior | Wound Healing | Ref |
|---|---|---|---|---|---|
| PCL-PLA/GelMA-ChMA | N.A. | N.A. | Fibroblasts cellular viability of ≈101.1% and ≈106.7%, for the top and bottom layer, respectively, after 7 days; After seven days, a continuous layer of cells with typical fibroblastic morphology and lamellipodia connecting to surrounding mesh was observed | N.A. | [55] |
| CS-PCL/HA | The optical density of E. coli was ≈0.09 for asymmetric membrane and ≈0.37 for the control (PCL), after 24 h of incubation | N.A. | Vero cells viability of ≈147.52%, after 5 days; Vero cells adhered to the asymmetric membrane showed a good cell–cell interaction as well as an improved cell/fibrous scaffold integration, after 5 days | N.A. | [26] |
| CS/PDLLA | N.A. | N.A. | Efficient attachment of fibroblast cells to the membrane, after 5 days of incubation; Infiltration of fibroblast cells into CS/PDLLA membranes up to a depth of ≈32.5 μm | The histology analysis, after 7 days of the wound treatment with the asymmetric membrane, demonstrated that the epidermis and dermis layer were gradually restored with the successful regeneration of keratinocytes and fibroblasts, respectively | [64] |
| BGB/BGA | N.A. | N.A. | The proliferation of fibroblast cells on the top and bottom layer was ≈184.41% and ≈208.68%, respectively, after 7 days; The proliferation of keratinocytes on the top and bottom layer was ≈190.18% and ≈183.74%, respectively, after 7 days | The asymmetric membranes induced the reduction of the wound size in ≈83.1% after 14 days; The histology analysis of the wound covered with the asymmetric membrane showed the re-epithelialization and a structure resembling the normal skin, with skin-like organized collagen fibers | [61] |
| PCL-mupirocin_ CS-LID | The membrane showed excellent activity against S. aureus (35 mm of inhibition zone), P. aeruginosa (30 mm of inhibition zone), and E. coli (28 mm of inhibition zone) | N.A. | The relative cell number (OD) of the fibroblast cells was ≈0.61 after 7 days | N.A. | [68] |
| PeCL(nylon mesh pore size 40)/Gel-pio | The top layer exhibited lower bacterial adhesion in comparison to the control, against S. aureus, E. coli, and P. aeruginosa | N.A. | The viabilities of fibroblast cells and HUVECs were ≈179.38% and ≈353.85%, respectively, after 3 days; The percentages of fibroblast cells and HUVECs migration were 61.54% and 68.57%, respectively | Type 2 Diabetic Mice: The wounds treated with PCL40/Gel-pio were almost completely closed on day 10, whereas the other groups needed more than 14 days; The blood glucose concentrations of the PCL40/Gel-pio group were maintained at a low level and increased after day 7, while the others increased continuously over time; Completely regenerated epidermis and dense dermis, and continuous and uniform granulation tissue on day 14; The relative collagen content on day 14 was 59.52%; The asymmetric membranes group showed the highest density of newly formed blood vessels (≈30.32 mature vessels per field) after 7 days; The asymmetric membranes group showed the most potent effect on cell proliferation (higher Ki67 expression, ≈73.23 positive cells per field) | [54] |
| Type 1 Diabetic Rat: Showed the fastest wound healing effect; The wounds treated with PCL40/Gel-pio were almost completely closed on day 14; Showed the higher density of collagen fibers (≈61.46%) after 7 days; Showed the highest density of newly formed blood vessels (≈32.27 vessels per field) after 5 days, and decreased to the day 14 (≈12.69 vessels per field); The asymmetric membranes group showed the most potent effect on cell proliferation (higher Ki67 expression, ≈56.55 positive cells per field), on day 14 | |||||
| PLA-VE/PCL-VE | N.A. | N.A. | Fibroblast cells viability of ≈87.44%, after 10 days; The surface of the membrane was highly colonized by fibroblast cells, and the cells’ attachment inside the pores of the membranes was also observed, after 10 days | After 14 days, the chick chorioallantoic membrane assay revealed the complete coverage of the asymmetric membrane with the newly formed vessels (≈48.99 blood vessels) | [57] |
| PCL/PVAc-CRV (Sample I-PVAc in DMF/ETOH; sample II-PVAc in DMF) | Sample I inhibited the proliferation of E. coli (from 1.6 × 109 to 1.2 × 107 CFU/mL) and S. aureus (5.7 × 1010 to 2.3 × 107 CFU/mL) Sample II inhibited the proliferation of E. coli (from 1.6 × 109 to 1.4 × 106) and S. aureus (5.7 × 1010 to 3.1 × 106) | N.A. | The fibroblast cells viability was ≈108.24% and ≈145.29% for sample I and sample II, respectively, after 3 days After 3 days, fibroblast cells properly adhered and spread homogeneously on the membrane; No significant effects on cells migration in in vitro wound closure assays, after 3 days | N.A. | [66] |
| PCL-HA/CS-ZN-SA | The asymmetric membranes showed an inhibitory effect of ≈99% against S. aureus and presented an inhibitory halo of 9.84 ± 3.64 mm | N.A. | The fibroblast cells viability was ≈106.05%, after 7 days; After 7 days, the cells presented filopodia protrusions and were completely attached on both layers | N.A. | [28] |
| PCL/PEO-CS-AV | Low bacteria adhesion to the upper side of the top layer; The asymmetric membranes showed antibacterial activity of 99.99% and 99.97% against S. aureus and E. coli, respectively | N.A. | The fibroblast cells viability was ≈94.44%, after 7 days; After 3 days, the fibroblast cells attached and proliferated; The fibroblasts migrated to the interior of the asymmetric membrane (8–10 µm within the polymeric structure), after 3 days | N.A. | [67] |
| PCL-SF/SF-HA-THY | The PCL-SF layer avoided the bacterial infiltration of S. aureus and P. aeruginosa; The SF-HA-THY layer showed antibacterial activity of 87.42% (and an inhibition zone of ≈69.90%) and 58.43% (and an inhibition zone of ≈52.38%), against S. aureus and P. aeruginosa, respectively | Antioxidant activity of 9.22% and ≈45.64% for the PCL-SF and SF-HA-THY membranes, respectively, after 8 h of incubation | The fibroblast cells viability was ≈93.44% and ≈93.82% for the PCL-SF and SF-HA-THY membranes, respectively, after 7 days; Both membranes promoted the cell adhesion, but in the SF_HA_THY layer, the fibroblast cells appeared to present more filopodia protrusions, higher cell adhesion, and proliferation | N.A. | [20] |
| PeCL/PDO-TiO2 nanoparticles (concentration of 3% (PP3T5T) and 5% (PP5T5T))-TTC | PP3T5T presented an inhibition zone of 12.78 ± 2.5 and 16.28 ± 4.7 µm, against S. aureus and E. coli, respectively; PP5T5T presented an inhibition zone of 26.14 ± 6.7 and 36.94 ± 5.6 µm, against S. aureus and E. coli, respectively | N.A. | The fibroblast cells proliferation was ≈107.77% and ≈110.83% for PP3T5T and PP5T5T, respectively, after 6 days; The fibroblast cells penetrated up to a depth of 40 and 35 µm for PP3T5T and PP5T5T, respectively, after 4 days | N.A. | [69] |
| PLLA-SS (1:1, 2:1, 4:1)-NFZ (0.2%, 0.5%, 1.0%)/NFZ-PLLA | Inhibition zones of 20.41 ± 0.43 to 24.28 ± 0.10 mm against E. coli and 21.47 ± 0.19 to 27.04 ± 0.35 mm against B. subtilis | N.A. | The fibroblast cells viabilities were all above 95% for the PLLA-SS(2:1)–0.2% NFZ (with concentrations ranging from 10 to 2.5 mg × mL−1), after 3 days | The asymmetric membranes induced the reduction of the wound size in 97% after 12 days | [71] |
| Aims | Composition | Biomolecule Incorporated | Layer of Incorporation | Encapsulation Efficiency and Loading Efficiency | Release Profile | Ref |
|---|---|---|---|---|---|---|
| Antibacterial activity | PCL/PVAc | CRV | Bottom layer | The CRV loaded in samples I and II was 3.0 ± 0.4 wt% and 2.3 ± 0.5 wt%, respectively | Sample I released about 45% of the total drug, while sample II released about 60% of the loaded CRV, at pH 8; after 7 days in basic pH the membranes were transferred to PBS pH 7.4 and after two weeks in this medium the release reached 60% and 85% of the loaded drug for samples I and II, respectively; after 14 days, the samples were put in an acidic medium where after one week the release reached 85% and 100% from the samples I and II, respectively | [66] |
| (Sample I—PVAc in DMF/ETOH; sample II—PVAc in DMF) | Encapsulation efficiencies were 55 ± 5% and 43 ± 9% for samples I and II, respectively | |||||
| PCL-HA/CS-ZN | SA | Bottom layer | N.A. | The release profile, in PBS (pH 5.5), consisted of a burst release in the first hour followed by a sustained release for 5 days (reaching approximately 16%) | [28] | |
| PCL/PEO-CS | AV | Bottom layer | N.A. | N.A. | [67] | |
| PCL-SF/SF-HA | THY | Bottom layer | Encapsulation efficiency of 79.7 ± 7.19% | THY release from the nanofibers, at both pH levels, comprises a burst release in the first 8 h after immersion in PBS, followed by a gradual release up to 24 h | [20] | |
| Loading efficiency of 64.8 ± 5.42% | At pH 8, the release of THY reached a maximum of 91.87 ± 0.99% | |||||
| At pH 5, the release of THY reached a maximum of 71.75 ± 2.06% | ||||||
| PeCL/PDO | TiO2 nanoparticles (concentration of 3% (PP3T5T) and 5% (PP5T5T)) and TTC | Bottom layer | N.A. | The release profile of TTC, in PBS (pH 7.4), from PP3T5T showed an initial burst release of 47.2% within the first 6 h, followed by a slow release that reached 61.9% until day 4 | [69] | |
| The burst release of TTC, in PBS (pH 7.4), from PPT5T5 was 50.8% within the first 6 h and reached 77% over 4 days | ||||||
| PLLA-SS/PLLA | NFZ | Both layers | N.A. | The top PLLA-SS nanofibrous mats with 0.2% of NFZ, in PBS (pH 7.4) presented a fast release profile with more than 98% of NFZ detected in 10 min of incubation for every ratio | [71] | |
| The PLLA bottom layer in PBS (pH 7.4) presented a more controlled and sustained release, reaching 17.6% after 48 h | ||||||
| PLLA-SS(2:1)-0.2NFZ/PLLA-2NFZ, PLLA-SS(2:1)-0.5NFZ/PLLA-2NFZ, and PLLA-SS(2:1)-1.0NFZ/PLLA-2NFZ in PBS (pH 7.4) presented a burst release of 11.2%, 14.3%, and 28.4%, respectively, and the release amounts reached 29.4%, 43.0%, and 53.9%, respectively, after 48 h | ||||||
| Wound healing improvement | PCL/CS | Mupirocin | Top layer | N.A. | The initial burst release of LID reached 66% in the first hours and increased gradually to 85% in the following 6 h, in PBS | [68] |
| LID | Bottom layer | The release of mupirocin consisted in the release of 57% of mupirocin in the first 6 h, followed by a sustained release (30% was released in the following 114 h), in PBS | ||||
| PeCL/Gel | Pio | Bottom layer | Loading efficiency of 56.16 ± 7.45% | The Pio release rapidly reached 40% in day 1 and a long-term release reached 75% in day 14, in PBS (pH 7.4) | [54] | |
| PLA/PCL | VE | Both layers | N.A. | The asymmetric membrane showed a sustained release of VE over 21 days reaching a maximum of 78%, in PBS | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, M.F.P.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics 2021, 13, 183. https://doi.org/10.3390/pharmaceutics13020183
Graça MFP, de Melo-Diogo D, Correia IJ, Moreira AF. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics. 2021; 13(2):183. https://doi.org/10.3390/pharmaceutics13020183
Chicago/Turabian StyleGraça, Mariana F. P., Duarte de Melo-Diogo, Ilídio J. Correia, and André F. Moreira. 2021. "Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review" Pharmaceutics 13, no. 2: 183. https://doi.org/10.3390/pharmaceutics13020183