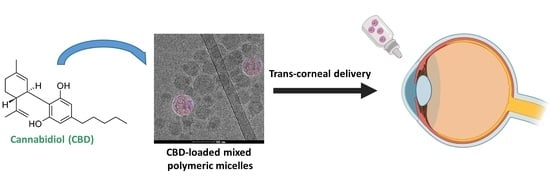
Cannabidiol-Loaded Mixed Polymeric Micelles of Chitosan/Poly(Vinyl Alcohol) and Poly(Methyl Methacrylate) for Trans-Corneal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Mixed Chitosan-g-Poly(Methyl Methacrylate):Poly(Vinyl Alcohol)-g-Poly(Methyl Methacrylate) Polymeric Micelles
2.2. Characterization of Mixed Chitosan-g-Poly(Methyl Methacrylate):Poly(Vinyl Alcohol)-g-Poly(Methyl Methacrylate) Polymeric Micelles
2.3. Compatibility of Mixed Polymeric Micelles in Human Corneal Epithelial Cells
2.4. Permeability of Mixed Polymeric Micelles across a Human Corneal Epithelium Model In Vitro
2.5. Statistical Analysis
3. Results and Discussion
3.1. Rationale
3.2. Production and Characterization of CBD-Loaded Mixed Polymeric Micelles
3.3. Drying and Redispersion of CBD-Loaded Mixed Polymeric Micelles
3.4. Compatibility of Mixed Polymeric Micelles with Human Cornea Epithelial Cells
3.5. Permeability of Mixed Polymeric Micelles across a Model of Corneal Epithelium In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef]
- Perucca, E. Cannabinoids in the Treatment of Epilepsy: Hard Evidence at Last? J. Epilepsy Res. 2017, 7, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Medical Cannabis Market Size, Trends & Growth|2021 to 2026. Available online: https://www.globenewswire.com/news-release/2020/07/14/2062165/0/en/Medical-Cannabis-Market-Size-Share-Growth-Analysis-Global-Trends-Industry-Overview-Regional-Forecast-by-2027-Top-Leaders-Aurora-Cannabis-Aphria-Medical-Cannabis-MedReleaf-Corp-Medi.html (accessed on 5 December 2021).
- Larsen, C.; Shahinas, J. Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. J. Clin. Med. Res. 2020, 12, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchel, D.; Stolar, O.; De-Haan, T.; Ziv-Baran, T.; Saban, N.; Fuchs, D.O.; Koren, G.; Berkovitch, M. Oral Cannabidiol Use in Children with Autism Spectrum Disorder to Treat Related Symptoms and Co-morbidities. Front. Pharmacol. 2019, 9, 1521. [Google Scholar] [CrossRef]
- Odi, R.; Bibi, D.; Wager, T.; Bialer, M. A perspective on the physicochemical and biopharmaceutic properties of marketed antiseizure drugs—From phenobarbital to cenobamate and beyond. Epilepsia 2020, 61, 1543–1552. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Domb, A.J.; Hoffman, A. The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. Eur. J. Pharm. Sci. 2017, 109, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Adelli, G.R.; Bhagav, P.; Taskar, P.; Hingorani, T.; Pettaway, S.; Gul, W.; ElSohly, M.A.; Repka, M.A.; Majumdar, S. Development of a Δ9-Tetrahydrocannabinol Amino Acid-Dicarboxylate Prodrug with Improved Ocular Bioavailability. Investig. Opthalmol. Vis. Sci. 2017, 58, 2167–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aebersold, A.; Duff, M.; Sloan, L.; Song, Z.-H. Cannabidiol Signaling in the Eye and Its Potential as an Ocular Therapeutic Agent. Cell. Physiol. Biochem. 2021, 55, 1–14. [Google Scholar]
- Rapino, C.; Tortolani, D.; Scipioni, L.; Maccarrone, L.S.A.M. Neuroprotection by (endo)Cannabinoids in Glaucoma and Retinal Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A. What is currently known about cannabidiol and ocular pressure. Expert Rev. Ophthalmol. 2019, 14, 259–261. [Google Scholar] [CrossRef] [Green Version]
- Tomida, I.; Pertwee, R.G.; Azuara-Blanco, A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004, 88, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Ahmed, I.; Patton, T.F. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Investig. Ophthalmol. Vis. Sci. 1985, 26, 584–587. [Google Scholar]
- Davies, N.M. Biopharmaceutical Considerations in Topical Ocular Drug Delivery. Clin. Exp. Pharmacol. Physiol. 2000, 27, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent Perspectives in Ocular Drug Delivery. Pharm. Res. 2008, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Votruba, A.R.; Farokhzad, O.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnik, A.; Mühlebach, S. Editorial: Drug Nanoparticles and Nano-Cocrystals: From Production and Characterization to Clinical Translation. Adv. Drug Deliv. Rev. 2018, 131, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Carcaboso, A. Nanomedicines in the future of pediatric therapy. Adv. Drug Deliv. Rev. 2014, 73, 140–161. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 10, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm. Res. 2006, 24, 1. [Google Scholar] [CrossRef]
- Sosnik, A. Temperature- and pH-sensitive polymeric micelles for drug encapsulation, release and targeting. In Smart Materials for Drug Delivery; Alvarez-Lorenzo, C., Concheiro, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; Chapter 5; pp. 115–147. [Google Scholar]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Moretton, M.A.; Glisoni, R.; Chiappetta, D.A.; Sosnik, A. Molecular implications in the nanoencapsulation of the anti-tuberculosis drug rifampicin within flower-like polymeric micelles. Colloids Surf. B 2010, 79, 467–479. [Google Scholar] [CrossRef]
- Raskin, M.M.; Schlachet, I.; Sosnik, A. Mucoadhesive nanogels by ionotropic crosslinking of chitosan-g-oligo(NiPAam) polymeric micelles as novel drug nanocarriers. Nanomedicine 2016, 11, 217–233. [Google Scholar] [CrossRef]
- Mahmud, A.; Xiong, X.-B.; Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug targeting. J. Drug Target. 2007, 15, 553–584. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Hocht, C.; Taira, C.; Sosnik, A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailaibility. Nanomedicine 2010, 5, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Sosnik, A.; Chiappetta, D.A.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Single and mixed poloxamine micelles as nanocarriers for solubilization and sustained release of ethoxzolamide for topical glaucoma therapy. J. R. Soc. Interface 2012, 9, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, D.A.; Hocht, C.; Opezzo, J.A.; Sosnik, A. Intranasal administration of antiretroviral-loaded micelles for anatomical targeting to the brain in HIV. Nanomedicine 2013, 8, 223–237. [Google Scholar] [CrossRef]
- Sosnik, A.; Raskin, M.M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnol. Adv. 2015, 33, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Glisoni, R.J.; Quintana L, S.S.; Molina, M.; Calderón, M.; Moglioni, A.G.; Sosnik, A. Chitosan-g-oligo(epsilon-caprolactone) polymeric micelles: Microwave-assisted synthesis and physicochemical and cytocompatibility characterization. J. Mater. Chem. B 2015, 3, 4853–4864. [Google Scholar] [CrossRef] [Green Version]
- Moshe, H.; Davizon, Y.; Raskin, M.M.; Sosnik, A. Novel poly(vinyl alcohol)-based amphiphilic nanogels by non-covalent boric acid crosslinking of polymeric micelles. Biomater. Sci. 2017, 5, 2295–2309. [Google Scholar] [CrossRef] [PubMed]
- Noi, I.; Schlachet, I.; Kumarasamy, M.; Sosnik, A. Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers 2018, 10, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlachet, I.; Trousil, J.; Rak, D.; Knudsen, K.D.; Pavlova, E.; Nyström, B.; Sosnik, A. Chitosan-graft-poly(methyl methacrylate) amphiphilic nanoparticles: Self-association and physicochemical characterization. Carbohydr. Polym. 2019, 212, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Halamish, H.M.; Trousil, J.; Rak, D.; Knudsen, K.D.; Pavlova, E.; Nyström, B.; Štěpánek, P.; Sosnik, A. Self-assembly and nanostructure of poly(vinyl alcohol)-graft-poly(methyl methacrylate) amphiphilic nanoparticles. J. Colloid Interface Sci. 2019, 553, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Schlachet, I.; Sosnik, A. Mixed Mucoadhesive Amphiphilic Polymeric Nanoparticles Cross a Model of Nasal Septum Epithelium in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 21360–21371. [Google Scholar] [CrossRef] [PubMed]
- Arzi, R.; Kay, A.; Raychman, Y.; Sosnik, A. Excipient-Free Pure Drug Nanoparticles Fabricated by Microfluidic Hydrodynamic Focusing. Pharmaceutics 2021, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Lazarjani, M.P.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for quantification of cannabinoids: A narrative review. J. Cannabis Res. 2020, 2, 35. [Google Scholar] [CrossRef]
- Rajendram, R.; Patel, V.B.; Preedy, V.R. Cannabis Neuropathology Resources and Recommended Reading. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1111–1113. [Google Scholar]
- Toropainen, E.; Ranta, V.P.; Talvitie, A.; Suhonen, P.; Urtti, A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2942–2948. [Google Scholar]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [Green Version]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.; Junginger, H. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, S91–S101. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef] [PubMed]
- Nepp, J.; Knoetzl, W.; Prinz, A.; Hoeller, S.; Prinz, M. Management of moderate-to-severe dry eye disease using chitosan-N-acetylcysteine (Lacrimera®) eye drops: A retrospective case series. Int. Ophthalmol. 2020, 40, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Pepić, I.; Lovrić, J.; Filipović-Grčić, J. How do polymeric micelles cross epithelial barriers? Eur. J. Pharm. Sci. 2013, 50, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, W. Biodegradation and biocompatibility of a degradable chitosan vascular prosthesis. Int. J. Clin. Exp. Med. 2015, 8, 3498–3505. [Google Scholar] [PubMed]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Nafee, N.A.; Boraie, N.A.; Ismail, F.A.; Mortada, L.M. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm. 2003, 53, 199–212. [Google Scholar] [PubMed]
- Gao, W.; Lai, J.C.K.; Leung, S.W. Functional enhancement of chitosan and nanoparticles in cell culture, tissue engineering, and pharmaceutical applications. Front. Physiol. 2012, 3, 321. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Schlachet, I. Innovative nano-biomaterials for the improved delivery of antitumorals to the central nervous system in the therapy of pediatric brain tumors. Ph.D. Thesis, Technion-Israel Institute of Technology, Haifa, Israel, December 2019. [Google Scholar]
- Bettencourt, A.; Almeida, A.J. Poly(methyl methacrylate) particulate carriers in drug delivery. J. Microencapsul. 2012, 29, 353–367. [Google Scholar] [CrossRef]
- Shaked, E.; Shani, Y.; Zilberman, M.; Scheinowitz, M. Poly(methyl methacrylate) particles for local drug delivery using shock wave lithotripsy: In vitro proof of concept experiment. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2015, 103, 1228–1237. [Google Scholar] [CrossRef]
- Gozum, N.; Unal, E.S.; Altan-Yaycioglu, R.; Gucukoglu, A.; Ozgun, C. Visual performance of acrylic and PMMA intraocular lenses. Eye 2003, 17, 238–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An Essential Material in Medicine and Dentistry. J. Autom. Inf. Sci. 2005, 15, 629–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Chen, G.; Nakamae, K.; Hoffman, A.S. An AB block copolymer of oligo(methyl methacrylate) and poly(acrylic acid) for micellar delivery of hydrophobic drugs. J. Control. Release 1998, 51, 221–229. [Google Scholar] [CrossRef]
- Shantha, K.L.; Harding, D.R.K. Synthesis and characterisation of chemically modified chitosan microspheres. Carbohydr. Polym. 2002, 48, 247–253. [Google Scholar] [CrossRef]
- Reguieg, F.; Ricci, L.; Bouyacoub, N.; Belbachir, M.; Bertoldo, M. Thermal characterization by DSC and TGA analyses of PVA hydrogels with organic and sodium MMT. Polym. Bull. 2019, 77, 929–948. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ng, W.B.; Bernt, W.; Cho, N.-J. Validation of Size Estimation of Nanoparticle Tracking Analysis on Polydisperse Macromolecule Assembly. Sci. Rep. 2019, 9, 2639. [Google Scholar]
- Mazzetti, C.; Ferri, E.; Pozzi, M.; Labra, M. Quantification of the content of cannabinol in commercially available e-liquids and studies on their thermal and photo-stability. Sci. Rep. 2020, 10, 3697. [Google Scholar]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Russo, F.; Linciano, P.; Strallhofer, S.S.; Tolomeo, F.; Forni, F.; Vandelli, M.A.; Gigli, G.; Cannazza, G. Origin of Δ9-Tetrahydrocannabinol Impurity in Synthetic Cannabidiol. Cannabis Cannabinoid Res. 2021, 6, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol—transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 2003, 93, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Coniglio, M.; Valenti, C.; Federici, M.I.; Lombardo, G.; Cianetti, S.; Marinucci, L. Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature. Biomed. Pharmacother. 2020, 132, 110728. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-E.; Basu, S.K.; Lee, V.H.L. Air-interface condition promotes the formation of tight corneal epithelial cell layers for drug transport studies. Pharm. Res. 2000, 17, 670–676. [Google Scholar] [CrossRef]
- Ben Shabo, R. Mucoadhesive amphiphilic polymeric micelles for ocular drug delivery. Master’s Thesis, Technion-Israel Institute of Technology, Haifa, Israel, October 2021. [Google Scholar]
- Anderson, J. Molecular Structure of Tight Junctions and Their Role in Epithelial Transport. Physiology 2001, 16, 126–130. [Google Scholar] [CrossRef]
- Schlachet, I.; Sosnik, A. Protoporphyrin IX-modified chitosan-g-oligo(NiPAAm) polymeric micelles: From physical stabilization to permeability characterization in vitro. Biomater. Sci. 2017, 5, 128–140. [Google Scholar] [CrossRef]






| Sample | Temperature (°C) | DLS | NTA | |||
|---|---|---|---|---|---|---|
| Dh–Intensity (nm) ± S.D. | PDI ± S.D. | Z-Potential (+mV) ± S.D. | Dh (nm) ± S.D. | Concentration (×109 Particles/mL)± S.D. | ||
| Non-crosslinked mixed PMs | 25 | 100 ± 10 | 0.30 ± 0.07 | +38 ± 2 | 114 ± 3 | 9.5 ± 0.3 |
| 37 | 96 ± 6 | 0.32 ± 0.07 | +33 ± 3 | 133 ± 1 | 3.9 ± 0.3 | |
| Crosslinked mixed PMs | 25 | 140 ± 20 | 0.38 ± 0.05 | +34 ± 4 | 129 ± 2 | 9.7 ± 0.4 |
| 37 | 140 ± 10 | 0.31 ± 0.07 | +32 ± 4 | 144 ± 4 | 3.6 ± 0.2 | |
| CBD-loaded non-crosslinked mixed PMs | 25 | 144 ± 6 | 0.21 ± 0.02 | +35 ± 2 | 151 ± 1 | 9.6 ± 0.1 |
| 37 | 142 ± 9 | 0.19 ± 0.02 | +33 ± 1 | 196 ± 2 | 3.4 ± 0.2 | |
| CBD-loaded crosslinked mixed PMs | 25 | 147 ± 9 | 0.19 ± 0.03 | +34 ± 3 | 120 ± 4 | 9.0 ± 0.7 |
| 37 | 151 ± 8 | 0.17 ± 0.02 | +33 ± 3 | 177 ± 4 | 3.9 ± 0.1 | |
| Sample | Drying Method | Dh–Intensity (nm) ± S.D. | PDI ± S.D. |
|---|---|---|---|
| Non-crosslinked mixed PMs | Freeze-drying | 113 ± 2 | 0.33 ± 0.02 |
| Spray-drying | 140 ± 10 | 0.41 ± 0.04 | |
| Crosslinked mixed PMs | Freeze-drying | 139 ± 4 | 0.36 ± 0.01 |
| Spray-drying | 135 ± 4 | 0.38 ± 0.03 | |
| CBD-loaded non-crosslinked mixed PMs | Freeze-drying | 130 ± 10 | 0.73 ± 0.03 |
| Spray-drying | 129 ± 6 | 0.30 ± 0.03 | |
| CBD-loaded crosslinked mixed PMs | Freeze-drying | 155 ± 8 | 0.62 ± 0.03 |
| Spray-drying | 142 ± 1 | 0.41 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosnik, A.; Shabo, R.B.; Halamish, H.M. Cannabidiol-Loaded Mixed Polymeric Micelles of Chitosan/Poly(Vinyl Alcohol) and Poly(Methyl Methacrylate) for Trans-Corneal Delivery. Pharmaceutics 2021, 13, 2142. https://doi.org/10.3390/pharmaceutics13122142
Sosnik A, Shabo RB, Halamish HM. Cannabidiol-Loaded Mixed Polymeric Micelles of Chitosan/Poly(Vinyl Alcohol) and Poly(Methyl Methacrylate) for Trans-Corneal Delivery. Pharmaceutics. 2021; 13(12):2142. https://doi.org/10.3390/pharmaceutics13122142
Chicago/Turabian StyleSosnik, Alejandro, Ronya Ben Shabo, and Hen Moshe Halamish. 2021. "Cannabidiol-Loaded Mixed Polymeric Micelles of Chitosan/Poly(Vinyl Alcohol) and Poly(Methyl Methacrylate) for Trans-Corneal Delivery" Pharmaceutics 13, no. 12: 2142. https://doi.org/10.3390/pharmaceutics13122142