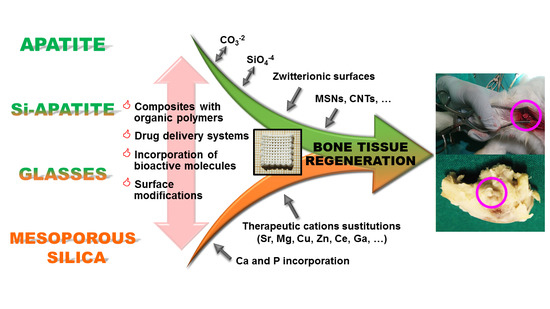
Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials
Abstract
:1. Introduction
- -
- It should possess a 3D interconnected porous hierarchical architecture.
- -
- It should possess a suitable surface chemistry and topography for cell attachment, proliferation and differentiation.
- -
- It should be tailored to include substances whose controlled release may contribute to the integration of the scaffolds without any adverse reactions.
- -
- It should be biocompatible and bioresorbable with controllable degradation and resorption rates to match tissue replacement without any undesirable by-products.
- -
- It should present mechanical properties that match those of the tissue during the reconstruction process. In addition, these scaffolds should be consistent enough to allow their manipulation during the cell seeding or surgical implantation procedures and even to be adapted in situ to fit odd-shaped defects.
- -
- It should show full reproducibility under large-scale manufacturing conditions.
- -
- Additionally, it should have a long shelf life and/or be straightforwardly preservable and be easily available to surgeons in a sterile operating environment.
2. Calcium Phosphate Scaffolds
3. Silicon-Substituted Hydroxyapatite (Si-HA) Scaffolds
4. Bioactive Glass (BG) Scaffolds
5. Mesoporus Bioactive Glass (MBG) Scaffolds
6. Mesoporous Silica Nanoparticles (MSNs) Scaffolds
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hollister, S.J. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I. Bioactive mesoporous silicas as controlled delivery systems: Application in bone tissue regeneration. J. Biomed. Nanotechnol. 2008, 4, 1–15. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Current trends on porous inorganic materials for biomedical applications. Chem. Eng. J. 2008, 137, 1–3. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2018, 84, 16–33. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Baeza, A.; Izquierdo-Barba, I.; Vallet-Regí, M. Biotinylation of silicon-doped hydroxyapatite: A new approach to protein fixation for bone tissue regeneration. Acta Biomater. 2010, 6, 743–749. [Google Scholar] [CrossRef]
- García, A.; Izquierdo-Barba, I.; Colilla, M.; de Laorden, C.L.; Vallet-Regí, M. Preparation of 3-D scaffolds in the SiO2–P2O5 system with tailored hierarchical meso-macroporosity. Acta Biomater. 2011, 7, 1265–1273. [Google Scholar] [CrossRef]
- Feito, M.J.; Lozano, D.; Alcaide, M.; Ramírez-Santillán, C.; Arcos, D.; Vallet-Regí, M.; Portolés, M.-T. Immobilization and bioactivity evaluation of FGF-1 and FGF-2 on powdered silicon-doped hydroxyapatite and their scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Electron. 2011, 22, 405–416. [Google Scholar] [CrossRef]
- Shruti, S.; Salinas, A.J.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. Acta Biomater. 2013, 9, 4836–4844. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Malmsten, M.; Doadrio, J.C.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Tailoring hierarchical meso–macroporous 3D scaffolds: From nano to macro. J. Mater. Chem. B 2014, 2, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Salcedo, S.; Shruti, S.; Salinas, A.J.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. In vitro antibacterial capacity and cytocompatibility of SiO2–CaO–P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. B 2014, 2, 4836–4847. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Portolés, P.; Montes-Casado, M.; Izquierdo-Barba, I.; Vallet-Regí, M.; Portolés, M.T. Effects of 3D nanocomposite bioceramic scaffolds on the immune response. J. Mater. Chem. B 2014, 2, 3469–3479. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. In vitro colonization of stratified bioactive scaffolds by pre-osteoblast cells. Acta Biomater. 2016, 44, 73–84. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Tomé, B.F.; Enciso, S.; Margallo, F.M.S.; Portolés, M.; et al. Mesoporous bioactive glass/ɛ-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater. 2019, 90, 393–402. [Google Scholar] [CrossRef]
- Gómez-Cerezo, M.N.; Lozano, D.; Arcos, D.; Vallet-Regí, M.; Vaquette, C. The effect of biomimetic mineralization of 3D-printed mesoporous bioglass scaffolds on physical properties and in vitro osteogenicity. Mater. Sci. Eng. C 2020, 109, 110572. [Google Scholar] [CrossRef]
- Heras, C.; Jiménez-Holguín, J.; Doadrio, A.; Vallet-Regí, M.; Sánchez-Salcedo, S.; Salinas, A. Multifunctional antibiotic- and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection. Acta Biomater. 2020, 114, 395–406. [Google Scholar] [CrossRef]
- Lozano, D.; Gil-Albarova, J.; Heras, C.; Sánchez-Salcedo, S.; Gómez-Palacio, V.E.; Gómez-Blasco, A.; Doadrio, J.C.; Vallet-Regí, M.; Salinas, A.J. ZnO-mesoporous glass scaffolds loaded with osteostatin and mesenchymal cells improve bone healing in a rabbit bone defect. J. Mater. Sci. Mater. Med. 2020, 31, 100. [Google Scholar] [CrossRef]
- Jiménez-Holguín, J.; López-Hidalgo, A.; Sánchez-Salcedo, S.; Peña, J.; Vallet-Regí, M.; Salinas, A.J. Strontium-Modified Scaffolds Based on Mesoporous Bioactive Glasses/Polyvinyl Alcohol Composites for Bone Regeneration. Materials 2020, 13, 5526. [Google Scholar] [CrossRef]
- Gómez-Cerezo, M.N.; Peña, J.; Ivanovski, S.; Arcos, D.; Vallet-Regí, M.; Vaquette, C. Multiscale porosity in mesoporous bioglass 3D-printed scaffolds for bone regeneration. Mater. Sci. Eng. C 2021, 120, 111706. [Google Scholar] [CrossRef]
- Cabañas, M.V.; Peña, J.; Román, J.; Vallet-Regí, M. Room temperature synthesis of agarose/sol–gel glass pieces with tailored interconnected porosity. J. Biomed. Mater. Res. Part A 2006, 78A, 508–514. [Google Scholar] [CrossRef]
- Román, J.; Cabañas, M.; Peña, J.; Doadrio, J.; Vallet-Regí, M. An optimized β-tricalcium phosphate and agarose scaffold fabrication technique. J. Biomed. Mater. Res. Part A 2008, 84A, 99–107. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Nieto, A.; Vallet-Regí, M. Hydroxyapatite/β-tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 62–71. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Balas, F.; Izquierdo-Barba, I.; Vallet-Regí, M. In vitro structural changes in porous HA/β-TCP scaffolds in simulated body fluid. Acta Biomater. 2009, 5, 2738–2751. [Google Scholar] [CrossRef]
- Cabañas, M.; Peña, J.; Román, J.; Vallet-Regí, M. Tailoring vancomycin release from β-TCP/agarose scaffolds. Eur. J. Pharm. Sci. 2009, 37, 249–256. [Google Scholar] [CrossRef]
- Alcaide, M.; Serrano, M.-C.; Román, J.; Cabañas, M.-V.; Peña, J.; Sánchez-Zapardiel, E.; Vallet-Regí, M.; Portolés, M.-T. Suppression of anoikis by collagen coating of interconnected macroporous nanometric carbonated hydroxyapatite/agarose scaffolds. J. Biomed. Mater. Res. Part A 2010, 95, 793–800. [Google Scholar] [CrossRef]
- Peña, J.; Román, J.; Cabañas, M.V.; Vallet-Regí, M. An alternative technique to shape scaffolds with hierarchical porosity at physiological temperature. Acta Biomater. 2010, 6, 1288–1296. [Google Scholar] [CrossRef]
- Román, J.; Cabañas, M.V.; Peña, J.; Vallet-Regí, M. Control of the pore architecture in three-dimensional hydroxyapatite-reinforced hydrogel scaffolds. Sci. Technol. Adv. Mater. 2011, 12, 045003. [Google Scholar] [CrossRef]
- Cabañas, M.V.; Pena, J.; Roman, J.; Ramírez-Santillán, C.; Matesanz, M.C.; Feito, M.J.; Portolés, M.T.; Vallet-Regí, M.; Cabañas, V. Design of tunable protein-releasing nanoapatite/hydrogel scaffolds for hard tissue engineering. Mater. Chem. Phys. 2014, 144, 409–417. [Google Scholar] [CrossRef]
- Paris, J.L.; Gomez, N.L.; Cabañas, M.V.; Román, J.; Peña, J.; Vallet-Regí, M. Fabrication of a nanoparticle-containing 3D porous bone scaffold with proangiogenic and antibacterial properties. Acta Biomater. 2019, 86, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Honduvilla, N.G.; Coca, A.; Ortega, M.A.; Trejo, C.; Román, J.; Peña, J.; Cabañas, V.; Regi, M.V.; Buján, J. Improved connective integration of a degradable 3D-nano-apatite/agarose scaffold subcutaneously implanted in a rat model. J. Biomater. Appl. 2018, 33, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Román, J.; Manzano, M.; Cabañas, M.V.; Vallet-Regí, M. Tuning dual-drug release from composite scaffolds for bone regeneration. Int. J. Pharm. 2015, 486, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Puértolas, J.A.; Vadillo, J.L.; Sanchez-Salcedo, S.; Nieto, A.; Gomez-Barrena, E.; Vallet-Regí, M. Compression behaviour of biphasic calcium phosphate and biphasic calcium phosphate–agarose scaffolds for bone regeneration. Acta Biomater. 2011, 7, 841–847. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Vila, M.; Vallet-Regí, M. Biological performance of hydroxyapatite–biopolymer foams: In vitro cell response. Acta Biomater. 2012, 8, 802–810. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Vila, M.; Izquierdo-Barba, I.; Cicuéndez, M.; Vallet-Regí, M. Biopolymer-coated hydroxyapatite foams: A new antidote for heavy metal intoxication. J. Mater. Chem. 2010, 20, 6956–6961. [Google Scholar] [CrossRef]
- Gil-Albarova, J.; Vila, M.; Badiola-Vargas, J.; Sánchez-Salcedo, S.; Herrera, A.; Vallet-Regi, M. In vivo osteointegration of three-dimensional crosslinked gelatin-coated hydroxyapatite foams. Acta Biomater. 2012, 8, 3777–3783. [Google Scholar] [CrossRef]
- Ardura, J.A.; Portal-Núñez, S.; Lozano, D.; Gutiérrez-Rojas, I.; Sánchez-Salcedo, S.; López-Herradón, A.; Mulero, F.; Villanueva-Peñacarrillo, M.L.; Vallet-Regí, M.; Esbrit, P. Local delivery of parathyroid hormone-related protein-derived peptides coated onto a hydroxyapatite-based implant enhances bone regeneration in old and diabetic rats. J. Biomed. Mater. Res. Part A 2016, 104, 2060–2070. [Google Scholar] [CrossRef] [Green Version]
- Lozano, D.; Sanchez-Salcedo, S.; Portal-Núñez, S.; Vila, M.; López-Herradón, A.; Ardura, J.A.; Mulero, F.; Gómez-Barrena, E.; Vallet-Regí, M.; Esbrit, P. Parathyroid hormone-related protein (107–111) improves the bone regeneration potential of gelatin–glutaraldehyde biopolymer-coated hydroxyapatite. Acta Biomater. 2014, 10, 3307–3316. [Google Scholar] [CrossRef] [Green Version]
- Sachs, E.; Cima, M.; Cornie, J. Three-Dimensional Printing: Rapid Tooling and Prototypes Directly from a CAD Model. CIRP Ann. 1990, 39, 201–204. [Google Scholar] [CrossRef]
- Petzold, R.; Zeilhofer, H.-F.; Kalender, W.A. Rapid prototyping technology in medicine—Basics and applications. Comput. Med. Imaging Graph. 1999, 23, 277–284. [Google Scholar] [CrossRef]
- Landers, R.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002, 23, 4437–4447. [Google Scholar] [CrossRef]
- Ang, T.H.; Sultana, F.S.A.; Hutmacher, D.W.; Wong, Y.S.; Fuh, J.Y.H.; Mo, X.M.; Loh, H.T.; Burdet, E.; Teoh, S.H. Fabrication of 3D chitosan–hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C 2002, 20, 35–42. [Google Scholar] [CrossRef]
- Qu, M.; Wang, C.; Zhou, X.; Libanori, A.; Jiang, X.; Xu, W.; Zhu, S.; Chen, Q.; Sun, W.; Khademhosseini, A. Multi-Dimensional Printing for Bone Tissue Engineering. Adv. Health Mater. 2021, 10, 2001986. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Heras, C.; Sanchez-Salcedo, S.; Lozano, D.; Peña, J.; Esbrit, P.; Vallet-Regi, M.; Salinas, A. Osteostatin potentiates the bioactivity of mesoporous glass scaffolds containing Zn2+ ions in human mesenchymal stem cells. Acta Biomater. 2019, 89, 359–371. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Gutierrez-Ríos, M.T.; Alonso, M.P.; De Frutos, M.I.; Nicolopoulos, S. Hydroxyapatite Particles Synthesized by Pyrolysis of an Aerosol. J. Solid State Chem. 1994, 112, 58–64. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.M.; Vallet-Regi, M. Controlled Crystallization of Calcium Phosphate Apatites. Chem. Mater. 2000, 12, 2460–2465. [Google Scholar] [CrossRef]
- Cabañas, M.V.; Vallet-Regí, M. Calcium phosphate coatings deposited by aerosol chemical vapour deposition. J. Mater. Chem. 2003, 13, 1104–1107. [Google Scholar] [CrossRef]
- Peña, J.; Vallet-Regí, M. Hydroxyapatite, tricalcium phosphate and biphasic materials prepared by a liquid mix technique. J. Eur. Ceram. Soc. 2003, 23, 1687–1696. [Google Scholar] [CrossRef]
- Hijón, N.; Cabañas, M.V.; Izquierdo-Barba, I.; Vallet-Regí, M. Bioactive Carbonate−Hydroxyapatite Coatings Deposited onto Ti6Al4V Substrate. Chem. Mater. 2004, 16, 1451–1455. [Google Scholar] [CrossRef]
- Peña, J.; Izquierdo-Barba, I.; Vallet-Regí, M. Calcium Phosphate Porous Coatings onto Alumina Substrates by Liquid Mix Method. In Key Engineering Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2004; Volume 254, pp. 359–362. [Google Scholar]
- Pena, J.; Izquierdo-Barba, I.; Garcia, M.A.; Vallet-Regi, M. Room temperature synthesis of chitosan/apatite powders and coatings. J. Eur. Ceram. Soc. 2006, 26, 3631–3638. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, S.; Werner, J.; Vallet-Regí, M. Hierarchical pore structure of calcium phosphate scaffolds by a combination of gel-casting and multiple tape-casting methods. Acta Biomater. 2008, 4, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Design and preparation of biocompatible zwitterionic hydroxyapatite. J. Mater. Chem. B 2013, 1, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. In vitro Evaluation of Potential Calcium Phosphate Scaffolds for Tissue Engineering. Tissue Eng. 2006, 12, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Fernández-Lanas, T.; González, B.; Vallet-Regí, M. Macroporous sol–gel hydroxyapatite moulding via confinement into shaped acrylate–acrylamide copolymers. J. Eur. Ceram. Soc. 2012, 32, 2121–2127. [Google Scholar] [CrossRef]
- Tang, D.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef]
- Xie, C.; Ye, J.; Liang, R.; Yao, X.; Wu, X.; Koh, Y.; Wei, W.; Zhang, X.; Ouyang, H. Advanced Strategies of Biomimetic Tissue-Engineered Grafts for Bone Regeneration. Adv. Health Mater. 2021, 10, 2100408. [Google Scholar] [CrossRef]
- Han, Y.; Wei, Q.; Chang, P.; Hu, K.; Okoro, O.V.; Shavandi, A.; Nie, L. Three-dimensional printing of hydroxyapatite com-posites for biomedical application. Crystals 2021, 11, 353. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R.P.; Jolly, S.S. Customized hydroxyapatites for bone-tissue engineering and drug delivery applications: A review. J. Sol-Gel Sci. Technol. 2020, 94, 505–530. [Google Scholar] [CrossRef]
- Fisher, S.A.; Tam, R.Y.; Shoichet, M.S. Tissue Mimetics: Engineered Hydrogel Matrices Provide Biomimetic Environments for Cell Growth. Tissue Eng. Part A 2014, 20, 895–898. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Padilla, S.; Izquierdo-Barba, I.; Vallet-Regí, M. High Specific Surface Area in Nanometric Carbonated Hydroxyapatite. Chem. Mater. 2008, 20, 5942–5944. [Google Scholar] [CrossRef]
- Morris, V.J.; Brownsey, G.J.; Chilvers, G.R.; Harris, J.E.; Gunning, A.P.; Ridout, M.J.; Stevens, B.J.H. Gelation of members of a family of branched anionic heteropolysaccharides produced by certain strains of Rhizobium leguminosarum. Carbohydr. Polym. 1990, 13, 165–183. [Google Scholar] [CrossRef]
- Tayalia, P.; Mooney, D.J. Controlled Growth Factor Delivery for Tissue Engineering. Adv. Mater. 2009, 21, 3269–3285. [Google Scholar] [CrossRef]
- Tessmar, J.K.; Göpferich, A.M. Matrices and scaffolds for protein delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 274–291. [Google Scholar] [CrossRef]
- Schieker, M.; Seitz, H.; Drosse, I.; Seitz, S.; Mutschler, W. Biomaterials as Scaffold for Bone Tissue Engineering. Eur. J. Trauma Emerg. Surg. 2006, 32, 114–124. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Lin, C.; Xiao, C.; Moseley, J.M.; Reid, I.R. Stimulation of Osteoblast Proliferation by C-Terminal Fragments of Parathyroid Hormone-Related Protein. J. Bone Miner. Res. 1999, 14, 915–922. [Google Scholar] [CrossRef]
- García-Martín, A.; Acitores, A.; Maycas, M.; Villanueva-Peñacarrillo, M.L.; Esbrit, P. Src kinases mediate VEGFR2 transactivation by the osteostatin domain of PTHrP to modulate osteoblastic function. J. Cell. Biochem. 2013, 114, 1404–1413. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Kleinheinz, J.; Stratmann, U.; Joos, U.; Wiesmann, H.-P. VEGF-Activated Angiogenesis During Bone Regeneration. J. Oral Maxillofac. Surg. 2005, 63, 1310–1316. [Google Scholar] [CrossRef]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2015, 83, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Sapkota, D.; Xue, Y.; Rajthala, S.; Yassin, M.A.; Finne-Wistrand, A.; Mustafa, K. Delivery of VEGFA in bone marrow stromal cells seeded in copolymer scaffold enhances angiogenesis, but is inadequate for osteogenesis as compared with the dual delivery of VEGFA and BMP2 in a subcutaneous mouse model. Stem Cell Res. Ther. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aderibigbe, B.; Sadiku, E.; Jayaramudu, J.; Ray, S.S. Controlled dual release study of curcumin and a 4-aminoquinoline analog from gum acacia containing hydrogels. J. Appl. Polym. Sci. 2015, 132, 41613. [Google Scholar] [CrossRef]
- Lee, J.S.; Bae, J.W.; Joung, Y.K.; Lee, S.J.; Han, D.K.; Park, K.D. Controlled dual release of basic fibroblast growth factor and indomethacin from heparin-conjugated polymeric micelle. Int. J. Pharm. 2008, 346, 57–63. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mouriño, V. Bisphosphonate-Based Strategies for Bone Tissue Engineering and Orthopedic Implants. Tissue Eng. Part B Rev. 2012, 18, 323–340. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Burkinshaw, R.; Winter, M.; Neville-Webbe, H.; Lester, J.; Woodward, E.; Brown, J. Zoledronic acid. Expert Opin. Drug Saf. 2010, 10, 133–145. [Google Scholar] [CrossRef]
- Rosenqvist, K.; Airaksinen, S.; Vehkamäki, M.; Juppo, A.M. Evaluating optimal combination of clodronate and bioactive glass for dental application. Int. J. Pharm. 2014, 468, 112–120. [Google Scholar] [CrossRef]
- Rainsford, K.D. Fifty years of ibuprofen: Advancing pain and fever management. Int. J. Clin. Pract. 2012, 67, 1–2. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Health Mater. 2020, 9, e2000310. [Google Scholar] [CrossRef]
- Boldbaatar, K.; Dashnyam, K.; Knowles, J.C.; Lee, H.-H.; Lee, J.-H.; Kim, H.-W. Dual-ion delivery for synergistic angiogenesis and bactericidal capacity with silica-based microsphere. Acta Biomater. 2019, 83, 322–333. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted Hydroxyapatites with Antibacterial Properties. BioMed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Yuan, Z.Y.; Huang, J. Substituted hydroxyapatite: A recent development. Mater. Technol. 2020, 35, 785–796. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gupta, A.; Verma, M.; Anand, M.P.; Sengupta, P.; Saravanan, M.; Manna, I.; Balani, K. Antibacterial and magnetic response of site-specific cobalt incorporated hydroxyapatite. Ceram. Int. 2020, 46, 513–522. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and Hydroxyapatite Based Biomaterials to Circumvent Periprosthetic Joint Infections. Materials 2021, 14, 804. [Google Scholar] [CrossRef]
- Lu, Y.; Li, M.; Li, L.; Wei, S.; Hu, X.; Wang, X.; Shan, G.; Zhang, Y.; Xia, H.; Yin, Q. High-activity chitosan/nano hydroxyapatite/zoledronic acid scaffolds for simultaneous tumor inhibition, bone repair and infection eradication. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 225–233. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Lei, Y.; Yin, W.-J.; Chen, Y.-X.; Ke, Q.-F.; Guo, Y.-P.; Zhang, C.-Q. Enhanced antibacterial activity and osteoinductivity of Ag-loaded strontium hydroxyapatite/chitosan porous scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 7919–7928. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Colilla, M.; González, B.; Vallet-Regí, M. Mesoporous silicananoparticles for the design of smart delivery nanodevices. Biomater. Sci. 2013, 1, 114–134. [Google Scholar] [CrossRef]
- Baeza, A.; Manzano, M.; Colilla, M.; Vallet-Regí, M. Recent advances in mesoporous silica nanoparticles for antitumor therapy: Our contribution. Biomater. Sci. 2016, 4, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for the Treatment of Complex Bone Diseases: Bone Cancer, Bone Infection and Osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashnyam, K.; Jin, G.-Z.; Kim, J.-H.; Perez, R.; Jang, J.-H.; Kim, H.-W. Promoting angiogenesis with mesoporous microcarriers through a synergistic action of delivered silicon ion and VEGF. Biomaterials 2017, 116, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: A Possible Factor in Bone Calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. In vivo Requirement for Silicon in Articular Cartilage and Connective Tissue Formation in the Chick. J. Nutr. 1976, 106, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Silicon: A requirement in bone formation independent of vitamin D1. Calcif. Tissue Int. 1981, 33, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. A Silicon Requirement for Normal Skull Formation in Chicks. J. Nutr. 1980, 110, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Marques, P.A.A.P.; Magalhães, M.C.F.; Correia, R.N.; Vallet-Regí, M. Synthesis and Characterisation of Silicon-Substituted Hydroxyapatite. Key Eng. Mater. 2000, 192–195, 247–250. [Google Scholar] [CrossRef]
- Balas, F.; Pérez-Pariente, J.; Vallet-Regí, M. In vitro bioactivity of silicon-substituted hydroxyapatites. J. Biomed. Mater. Res. Part A 2003, 66, 364–375. [Google Scholar] [CrossRef]
- Arcos, D.; Rodríguez-Carvajal, J.; Vallet-Regí, M. The effect of the silicon incorporation on the hydroxylapatite structure. A neutron diffraction study. Solid State Sci. 2004, 6, 987–994. [Google Scholar] [CrossRef]
- Arcos, D.; Rodríguez-Carvajal, J.; Vallet-Regí, M. Silicon Incorporation in Hydroxylapatite Obtained by Controlled Crystallization. Chem. Mater. 2004, 16, 2300–2308. [Google Scholar] [CrossRef]
- Arcos, D.; Rodríguez-Carvajal, J.; Vallet-Regí, M. Crystal-Chemical Characteristics of Silicon−Neodymium Substituted Hydroxyapatites Studied by Combined X-ray and Neutron Powder Diffraction. Chem. Mater. 2004, 17, 57–64. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Arcos, D. Silicon substituted hydroxyapatites. A method to upgrade calcium phosphate based implants. J. Mater. Chem. 2005, 15, 1509–1516. [Google Scholar] [CrossRef]
- Arcos, D.; Sánchez-Salcedo, S.; Izquierdo-Barba, I.; Ruiz, L.; González-Calbet, J.; Vallet-Regí, M. Crystallochemistry, textural properties, and in vitro biocompatibility of different silicon-doped calcium phosphates. J. Biomed. Mater. Res. Part A 2006, 78A, 762–771. [Google Scholar] [CrossRef]
- Manzano, M.; Lozano, D.; Arcos, D.; Portal-Núñez, S.; la Orden, C.L.; Esbrit, P.; Vallet-Regí, M. Comparison of the osteoblastic activity conferred on Si-doped hydroxyapatite scaffolds by different osteostatin coatings. Acta Biomater. 2011, 7, 3555–3562. [Google Scholar] [CrossRef]
- Vila, M.; García, A.; Girotti, A.; Alonso, M.; Rodríguez-Cabello, J.C.; González-Vázquez, A.; Planell, J.A.; Engel, E.; Buján, J.; García-Honduvilla, N.; et al. 3D silicon doped hydroxyapatite scaffolds decorated with Elastin-like Recombinamers for bone regenerative medicine. Acta Biomater. 2016, 45, 349–356. [Google Scholar] [CrossRef]
- Matesanz, M.C.; Linares, J.; Oñaderra, M.; Feito, M.J.; Martínez-Vázquez, F.J.; Sánchez-Salcedo, S.; Arcos, D.; Portolés, M.T.; Vallet-Regí, M. Response of osteoblasts and preosteoblasts to calcium deficient and Si substituted hydroxyapatites treated at different temperatures. Colloids Surf. B Biointerfaces 2015, 133, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Casarrubios, L.; Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Feito, M.J.; Serrano, M.C.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Tomé, B.F.; et al. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater. 2020, 101, 544–553. [Google Scholar] [CrossRef]
- Martínez-Vázquez, F.J.; Cabañas, M.V.; Paris, J.L.; Lozano, D.; Vallet-Regí, M. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015, 15, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Meseguer-Olmo, L.; Vicente-Ortega, V.; Alcaraz-Baños, M.; Calvo-Guirado, J.L.; Vallet-Regí, M.; Arcos, D.; Baeza, A. In-vivo behavior of Si-hydroxyapatite/polycaprolactone/DMB scaffolds fabricated by 3D printing. J. Biomed. Mater. Res. Part A 2013, 101A, 2038–2048. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 1210–1219. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; De Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [Green Version]
- Kelpke, S.S.; Zinn, K.R.; Rue, L.W.; Thompson, J.A. Site-specific delivery of acidic fibroblast growth factor stimulates angiogenic and osteogenic responsesin vivo. J. Biomed. Mater. Res. 2004, 71A, 316–325. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef]
- Jonca, F.; Ortéga, N.; Gleizes, P.-E.; Bertrand, N.; Plouët, J. Cell Release of Bioactive Fibroblast Growth Factor 2 by Exon 6-encoded Sequence of Vascular Endothelial Growth Factor. J. Biol. Chem. 1997, 272, 24203–24209. [Google Scholar] [CrossRef] [Green Version]
- Urry, D.W.; Parker, T.M. Mechanics of elastin: Molecular mechanism of biological elasticity and its relationship to contraction. J. Muscle Res. Cell Motil. 2002, 23, 543–559. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; Arias, F.J.; Rodrigo, M.A.; Girotti, A. Elastin-like polypeptides in drug delivery. Adv. Drug Deliv. Rev. 2016, 97, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gu, J.; Zhang, Y.; Tan, Y.; Zhou, J.; Zhou, D. Immobilization of RGD peptide onto the surface of apatite-wollastonite ceramic for enhanced osteoblast adhesion and bone regeneration. J. Wuhan Univ. Technol. Sci. Ed. 2014, 29, 626–634. [Google Scholar] [CrossRef]
- Sitasuwan, P.; Lee, L.A.; Li, K.; Nguyen, H.G.; Wang, Q. RGD-conjugated rod-like viral nanoparticles on 2D scaffold improve bone differentiation of mesenchymal stem cells. Front. Chem. 2014, 2, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.S.; Hay, D.I.; Schluckebier, S.K. Inhibition of calcium phosphate precipitation by human salivary statherin: Structure-activity relationships. Calcif. Tissue Int. 1992, 50, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Stayton, P.S.; Drobny, G.P.; Shaw, W.J.; Long, J.R.; Gilbert, M. Molecular Recognition at the Protein-Hydroxyapatite Interface. Crit. Rev. Oral Biol. Med. 2003, 14, 370–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, S.; Shkilnyy, A.; Rumplasch, C.; Ribeiro, A.; Arias, F.J.; Rodríguez-Cabello, J.C.; Taubert, A. Biomimetic Calcium Phosphate Mineralization with Multifunctional Elastin-Like Recombinamers. Biomacromolecules 2011, 12, 1480–1486. [Google Scholar] [CrossRef]
- Raj, P.D.; Johnsson, M.; Levine, M.J.; Nancollas, G.H. Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J. Biol. Chem. 1992, 267, 5968–5976. [Google Scholar] [CrossRef]
- Hao, J.; Yuan, M.; Deng, X. Biodegradable and biocompatible nanocomposites of poly(ε-caprolactone) with hydroxyapatite nanocrystals: Thermal and mechanical properties. J. Appl. Polym. Sci. 2002, 86, 676–683. [Google Scholar] [CrossRef]
- Prabhakar, R.L.; Brocchini, S.; Knowles, J.C. Effect of glass composition on the degradation properties and ion release characteristics of phosphate glass—Polycaprolactone composites. Biomaterials 2005, 26, 2209–2218. [Google Scholar] [CrossRef]
- Erdemli, O.; Çaptug, O.; Bilgili, H.; Orhan, D.; Tezcaner, A.; Keskin, D. In vitro and in vivo evaluation of the effects of demineralized bone matrix or calcium sulfate addition to polycaprolactone–bioglass composites. J. Mater. Sci. Mater. Electron. 2021, 21, 295–308. [Google Scholar] [CrossRef]
- Hoque, E.; San, W.Y.; Wei, F.; Li, S.; Huang, M.-H.; Vert, M.; Hutmacher, D.W. Processing of Polycaprolactone and Polycaprolactone-Based Copolymers into 3D Scaffolds, and Their Cellular Responses. Tissue Eng. Part A 2009, 15, 3013–3024. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell culture response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Niehaus, A.J.; Anderson, D.; Samii, V.F.; Weisbrode, S.E.; Johnson, J.K.; Noon, M.S.; Tomasko, D.L.; Lannutti, J.J. Effects of orthopedic implants with a polycaprolactone polymer coating containing bone morphogenetic protein-2 on osseointegration in bones of sheep. Am. J. Vet. Res. 2009, 70, 1416–1425. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. Part A 2010, 94, 241–251. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- McMillan, J.; Kinney, R.C.; Ranly, D.M.; Fatehi-Sedeh, S.; Schwartz, Z.; Boyan, B.D. Osteoinductivity of demineralized bone matrix in immunocompromised mice and rats is decreased by ovariectomy and restored by estrogen replacement. Bone 2007, 40, 111–121. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Inorganic nanotubes reinforced polyvinylidene fluoride composites as low-cost electromagnetic interference shielding materials. Nanoscale Res. Lett. 2011, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Haas, T.W.; Guiseppi-Elie, A.; Bowlin, G.L.; Simpson, D.G.; Wnek, G.E. Electrospinning and Stabilization of Fully Hydrolyzed Poly(Vinyl Alcohol) Fibers. Chem. Mater. 2003, 15, 1860–1864. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, X.; Sheng, J. Immobilization of cellulase in nanofibrous PVA membranes by electrospinning. J. Membr. Sci. 2005, 250, 167–173. [Google Scholar] [CrossRef]
- Alexandre, N.; Ribeiro, J.; Gartner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A.; et al. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting-In vitroandin vivostudies. J. Biomed. Mater. Res. Part A 2014, 102, 4262–4275. [Google Scholar] [CrossRef] [PubMed]
- Akkineni, A.R.; Luo, Y.; Schumacher, M.; Nies, B.; Lode, A.; Gelinsky, M. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater. 2015, 27, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.; Fernández-Villa, D.; Serrano, M.C.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Margallo, F.M.S.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ragel, C.V.; Salinas, A. Glasses with Medical Applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Padilla, S.; Sánchez-Salcedo, S.; Vallet-Regí, M. Bioactive glass as precursor of designed-architecture scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2006, 81A, 224–232. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Perez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2000, 13, 308–311. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly Ordered Mesoporous Bioactive Glasses with Superior In Vitro Bone-Forming Bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- López-Noriega, A.; López de Laorden, C.; Arcos, D.; Vallet-Regí, M. Fabricación de Andamios Tridimensionales con Vidrios Meso-Porosos Bioactivos Mediante Prototipado Rápido. Spanish Patent No. P201000353, 21 November 2012. [Google Scholar]
- Schierholz, J.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Salinas, A.J. Mesoporous bioactive glasses for regenerative medicine. Mater. Today Bio 2021, 11, 100121. [Google Scholar] [CrossRef]
- Wang, N.; Dheen, S.T.; Fuh, J.Y.H.; Kumar, A.S. A review of multi-functional ceramic nanoparticles in 3D printed bone tissue engineering. Bioprinting 2021, 23, e00146. [Google Scholar] [CrossRef]
- Colilla, M.; Vallet-Regí, M. Targeted Stimuli-Responsive Mesoporous Silica Nanoparticles for Bacterial Infection Treatment. Int. J. Mol. Sci. 2020, 21, 8605. [Google Scholar] [CrossRef]
- Paris, J.L.; Vallet-Regí, M. Ultrasound-Activated Nanomaterials for Therapeutics. Bull. Chem. Soc. Jpn. 2020, 93, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Manzano, M.; Vallet-Regí, M. Revisiting bioceramics: Bone regenerative and local drug delivery systems. Prog. Solid State Chem. 2012, 40, 17–30. [Google Scholar] [CrossRef]
- Mas, N.; Arcos, D.; Polo, L.; Aznar, E.; Sánchez-Salcedo, S.; Sancenón, F.; García, A.; Marcos, M.D.; Baeza, A.; Vallet-Regí, M.; et al. Towards the Development of Smart 3D “Gated Scaffolds” for On-Command Delivery. Small 2014, 10, 4859–4864. [Google Scholar] [CrossRef]









| Scaffold Type | Organic Polymer (Binder Agent) * | Other Subsequent Incorporations | Effects | Ref. |
|---|---|---|---|---|
| Si-HA | Hydroxypropyl methylcellulose ** | Higher surface areas and porosities that facilitate protein adsorption (albumin and fibrinogen) | [117] | |
| MMA+MBAA ** | Surface functionalization with biotin by covalent bonding | Possibility of further anchoring of more molecules from the biotin present on the scaffold surfaces that could act as a linker | [7] | |
| Osteostatin incorporation by adsorption or covalently anchored | Regardless of the osteostatin incorporation strategy, its presence stimulates preosteoblast cell growth and matrix mineralization | [115] | ||
| Fibroblast growth factors (FGFs) adsorption | Improve adhesion and proliferation of osteoblastic cells. | [9] | ||
| Elastin-like recombinamers (ELRs) adsorption. | Induce bone marrow mesenchymal stromal cells proliferation and differentiation into osteoblastic lineage | [116] | ||
| NanoSi-HA | PVA *** | Vascular endothelial growth factor (VEGF) adsorption. | Poor results but VEGF presence increased volume of newly formed bone, trabeculae thickness and implant vascularization in sheep in vivo model | [118] |
| Si-HA/Gelatine | Gelatine type A | Vancomycin | Improve pre-osteoblatic cells differentiation and ALP gene expression. Cargo-antibiotic agent for drug delivery. | [119] |
| Si-HA/PCL | PCL | Good results of biocompatibility, osteroconductive features and new bone formation capability in in vivo New Zealand rabbit’s studies | [120] | |
| Si-HA/PCL/DBM | Incorporation of demineralized bone matrix (DMB) | In vivo New Zealand rabbit’s studies: new bone formation in the peripheral portions of the scaffolds and within its pores. | ||
| Si-HA/PCL/CNTs | Incorporation of carbon nanotubes (CNTs) | Improve protein adsorption and osteoblast-like cells attachment. | [121] | |
| Si-HA/PVA | PVA | Vascular endothelial growth factor (VEGF) adsorption. | Stimulated endothelial (EC2) proliferation and pre-osteoblasts (MC3T3-E1) differentiation | [118] |
| Scaffold Type | Organic Polymer (Binder Agent) | Scaffold Modification | Effects | Ref. |
|---|---|---|---|---|
| (a) MGHA | Hydroxy methylcellulose | Nano HA embedded and amine functionalization | Enhanced preosteoblast adhesion, proliferation and differentiation | [11] |
| MBG/PCL | PCL | PBS particles | Extra microporous Increase bioactivity and neovascularization | [22] |
| Zoledronic acid loaded | Antiresorptive and avoids inflammatory response | [17] | ||
| (b) 4Zn-MBG * | PCL/Gelatine crosslinked GA | Osteostatin | Osteogenic | [20,47] |
| Osteostatin and MSCs | Significantly improved trabecular bone volume density from μCT | [20] | ||
| (a) MGHA | Hydroxy methylcelullose | Antibiotic loading (Levofloxacin) | pH-dependent Levofloxacin release is able to inhibit the S. aureus growth and to destroy a preformed biofilm | [16] |
| GRIFMGLEVPVAVAN | PVA | Antibiotic loading (Levofloxacin, Rifanpicin, Vancomicin) | Multidrug scaffolds release | [15] |
| (b) X-MBG * X = Ce, Ga, Zn, Sr | PCL and/or Gelatine crosslinked GA | Therapeutic ions | Osteogenic, angiogenic and antimicrobial | [10,19,21] |
| (b) 4Zn-MBG * | PCL/Gelatine crosslinked GA | Antibiotic loading (Levofloxacin, Rifanpicin, Vancomicin and Gentamicin) | Eliminates Staphylococcus and Escherichia biofilms and inhibits bacteria growth in very short time periods | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, A.; Cabañas, M.V.; Peña, J.; Sánchez-Salcedo, S. Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials. Pharmaceutics 2021, 13, 1981. https://doi.org/10.3390/pharmaceutics13111981
García A, Cabañas MV, Peña J, Sánchez-Salcedo S. Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials. Pharmaceutics. 2021; 13(11):1981. https://doi.org/10.3390/pharmaceutics13111981
Chicago/Turabian StyleGarcía, Ana, María Victoria Cabañas, Juan Peña, and Sandra Sánchez-Salcedo. 2021. "Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials" Pharmaceutics 13, no. 11: 1981. https://doi.org/10.3390/pharmaceutics13111981