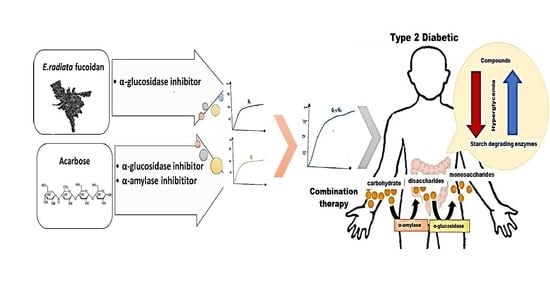
A Combination Approach in Inhibiting Type 2 Diabetes-Related Enzymes Using Ecklonia radiata Fucoidan and Acarbose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Harvesting and Preparation of Seaweeds
2.2. Fucoidan Extraction
2.3. Structural Analysis of Extracted Fucoidan
2.3.1. Fourier Transform Infrared Spectrometer (FT-IR) Analysis
2.3.2. NMR Spectroscopy Analysis
2.3.3. Thermogravimetric Analysis
2.4. Chemical Composition Analysis of Fucoidan
2.5. Determination of Average Molecular Weight
2.6. Carbohydrate Digesting Enzymes Inhibition Studies
2.6.1. α-Amylase Activity Assay
2.6.2. α-Glucosidase Activity Assay
2.7. Interaction between Fucoidan and α-Glucosidase
2.7.1. Tryptophan Fluorescence Analysis of the α-Glucosidase-Fucoidan Interaction
2.7.2. Determination of Binding Parameters of the α-Glucosidase-Fucoidan Interaction
2.7.3. Circular Dichroism Analysis of Secondary Structural Changes of α-Glucosidase upon Interaction with E. radiata Fucoidan
2.7.4. Mode of α-Glucosidase Inhibition
2.8. Investigating the Synergistic Potential of Extracted Fucoidan with Acarbose
2.9. Fucoidan Toxicity Screening
2.9.1. Cell Culture
2.9.2. Resazurin Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Fucoidan Yield
3.2. Structural Validation of E. radiata Fucoidan
3.2.1. FT-IR Spectroscopy Analysis
3.2.2. 1H NMR Analysis
3.2.3. Thermogravimetric Analysis
3.3. Chemical Profiling and Molecular Size Estimation of E. radiata Fucoidan
3.4. Inhibition of Carbohydrate Digestion Enzymes
3.4.1. Inhibition of α-Amylase by Acarbose
3.4.2. Inhibition of α-Glucosidase
3.4.3. Fucoidan Directly Interacts with α-Glucosidase
3.4.4. Mode of Inhibition of α-Glucosidase Activity by E. radiata Fucoidan
3.5. A Poly Compound (Acarbose and Fucoidan) Combination Approach in Inhibiting α-Glucosidase
3.6. Cytotoxicity of Fucoidan
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 18 March 2021).
- Moini, J. The Epidemic and Prevalence of Diabetes in the United States. In Epidemiology of Diabetes; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 45–55. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Cho, M.; Han, J.H.; You, S. Inhibitory effects of fucan sulfates on enzymatic hydrolysis of starch. LWT 2011, 44, 1164–1171. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Kumar, T.V.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan. A α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2016, 22, 56. [Google Scholar] [CrossRef] [Green Version]
- McIver, L.A.; Tripp, J. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493214/ (accessed on 18 March 2021).
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. α-amylase and α-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Yang, X.-D.; Liu, C.-G.; Tian, Y.-J.; Gao, D.-H.; Li, W.-S.; Ma, H.-L. Inhibitory effect of fucoidan on hypoglycemia in diabetes mellitus anim. Int J Clin Exp Med. 2017, 10, 8529–8534. [Google Scholar]
- Bolton, J.J.; Stegenga, H. Seaweed species diversity in South Africa. South Afr. J. Mar. Sci. 2002, 24, 9–18. [Google Scholar] [CrossRef]
- Morán-Santibañez, K.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Robledo, D.; Freile-Pelegrin, Y.; Peña-Hernández, M.A.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Synergistic Effects of Sulfated Polysaccharides from Mexican Seaweeds against Measles Virus. BioMed Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Greco, W.R.; Faessel, H.; Levasseur, L. The Search for Cytotoxic Synergy Between Anticancer Agents: A Case of Dorothy and the Ruby Slippers? J. Natl. Cancer Inst. 1996, 88, 699–700. [Google Scholar] [CrossRef] [Green Version]
- Roell, K.R.; Reif, D.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy—Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Chou, T.T.C. Compusyn. Available online: http://www.Combosyn.com (accessed on 10 January 2020).
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R.; Gunasekaran, P.; Kannan, S.; Palani, P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process. Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Mabate, B.; Zininga, T.; Ramatsui, L.; Makumire, S.; Achilonu, I.; Dirr, H.; Shonhai, A. Structural and biochemical characterization of Plasmodium falciparum Hsp70-x reveals functional versatility of its C-terminal EEVN motif. Proteins: Struct. Funct. Bioinform. 2018, 86, 1189–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zininga, T.; Achilonu, I.; Hoppe, H.; Prinsloo, E.; Dirr, H.; Shonhai, A. Overexpression, Purification and Characterisation of the Plasmodium falciparum Hsp70-z (PfHsp70-z) Protein. PLoS ONE 2015, 10, e0129445. [Google Scholar] [CrossRef] [Green Version]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2007, 89, 392–400. [Google Scholar] [CrossRef]
- January, G.; Naidoo, R.; Kirby-McCullough, B.; Bauer, R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019, 40, 101517. [Google Scholar] [CrossRef]
- Fernando, S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; De Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. ALGAE 2017, 32, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industries. Int. J. Carbohydr. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B.; Mejías, E.; Moenne, A. Alginic acids in Lessonia vadosa: Partial hydrolysis and elicitor properties of the polymannuronic acid fraction. Environ. Boil. Fishes 2004, 16, 127–133. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural Characterization of Fucoidan from Laminaria hyperborea: Assessment of Coagulation and Inflammatory Properties and Their Structure–Function Relationship. ACS Appl. Bio Mater. 2018, 1, 1880–1892. [Google Scholar] [CrossRef] [Green Version]
- Alwarsamy, M.; Gooneratne, R.; Ravichandran, R. Effect of fucoidan from Turbinaria conoides on human lung adenocarcinoma epithelial (A549) cells. Carbohydr. Polym. 2016, 152, 207–213. [Google Scholar] [CrossRef]
- Thangapandi, M.; Kumar, A.T.T. Effect of fucoidan from Turbinaria ornata against marine ornamental fish pathogens. J. Coast. Life Med. 2013, 1, 282–286. [Google Scholar]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Liu, X.; Yu, W. Evaluating the thermal stability of high performance fibers by TGA. J. Appl. Polym. Sci. 2006, 99, 937–944. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Methacanon, P.; Franco, C.; Su, P.; Zhang, W. Gut health benefits of brown seaweed Ecklonia radiata and its polysaccharides demonstrated in vivo in a rat model. J. Funct. Foods 2017, 37, 676–684. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [Green Version]
- Kotowaroo, M.I.; Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Screening of Traditional Anti-diabetic Medicinal Plants of Mauritius for Possible α-Amylase Inhibitory Effects in vitro. Phytother.Res. 2006, 20, 228–231. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, W.; Wei, J.; Lin, X. Synthesis and α-Glucosidase Inhibitory Mechanisms of Bis(2,3-dibromo-4,5-dihydroxybenzyl) Ether, a Potential Marine Bromophenol α-Glucosidase Inhibitor. Mar. Drugs 2011, 9, 1554–1565. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Wang, L.; Niesen, D.B.; Cai, A.; Cho, B.P.; Tan, W.; Gu, Q.; Xu, J.; Seeram, N.P. Structure activity related, mechanistic, and modeling studies of gallotannins containing a glucitol-core and α-glucosidase. RSC Adv. 2015, 5, 107904–107915. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S.; Özel, A.; Barut, B. α-Glucosidase inhibitory effect of Potentilla astracanica and some isoflavones: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Int. J. Biol. Macromol. 2017, 105, 1062–1070. [Google Scholar] [CrossRef]
- Lopina, O.D. Enzyme inhibitors and activators. In Enzyme Inhibitors and Activators; Senturk, M., Ed.; IntechOpen: London, UK, 2017; p. 247. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Goldman, L.; Bennett, J.C. Disorders of gastrointestinal motility. In Cecil Medicine, 26th ed.; Goldman, L., Schafer, A.I., Eds.; Saunders Co: Philadelphia, PA, USA, 2000. [Google Scholar]
- Stauffer, J.S.; Manzano, L.A.; Balch, G.C.; Merriman, R.L.; Tanzer, L.R.; Moyer, M.P. Development and characterization of normal colonic epithelial cell lines derived from normal mucosa of patients with colon cancer. Am. J. Surg. 1995, 169, 190–196. [Google Scholar] [CrossRef]








| Components | w/w % SD |
|---|---|
| Total carbohydrate a | 88.1 ± 7.4 |
| Total reducing sugars b | 50.9 ± 10.3 |
| Total phenolics c | 1.9 ± 0.4 |
| Sulphate content d | 8.8 ± 1.4 |
| Uronic acids e | 2.2 ± 0.7 |
| Total protein f | 2.3 ± 0.89 |
| L-fucose g | 3.7 ± 0.43 |
| Glucose g | 7.31 ± 0.64 |
| Galactose g | 4.9 ± 1.2 |
| Mannose g | 4.23 ± 0.22 |
| Arabinose g | ND |
| Fructose g | ND |
| Ash content h | 15.5 |
| Mw (kDa) i | >100 |
| Inhibitor | IC50 of Fucoidan/Compound (µg/mL) |
|---|---|
| E. radiata | 19 |
| F. vesiculosus control | 16 |
| Acarbose control | 332 |
| Points on Isobologram | Compounds Combinations | Compound Concentration (µg/mL) Acarbose: E. radiata | % Residual α-Glucosidase Activity ± SD | CI | Effect | |
|---|---|---|---|---|---|---|
| 1 | IC75-IC75 | 2223 | 35.2 | 18.1 ± 2.35 | 0.63 | Synergistic |
| 2 | IC75-IC50 | 2223 | 18.6 | 26.1 ± 3.64 | 0.68 | Synergistic |
| 3 | IC75-IC25 | 2223 | 9.9 | 39.8 ± 2.96 | 0.98 | Synergistic |
| 4 | IC50-IC75 | 922 | 35.2 | 20.1 ± 2.56 | 0.58 | Synergistic |
| 5 | IC50-IC50 | 922 | 18.6 | 26.8 ± 3.4 | 0.51 | Synergistic |
| 6 | IC50-IC25 | 922 | 9.9 | 47.7 ± 6.39 | 0.83 | Synergistic |
| 7 | IC25-IC75 | 382 | 35.2 | 20.6 ± 3.41 | 0.52 | Synergistic |
| 8 | IC25-IC50 | 382 | 18.6 | 29.6 ± 3.72 | 0.49 | Synergistic |
| 9 | IC25-IC25 | 382 | 9.9 | 51.4 ± 7.2 | 0.68 | Synergistic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. A Combination Approach in Inhibiting Type 2 Diabetes-Related Enzymes Using Ecklonia radiata Fucoidan and Acarbose. Pharmaceutics 2021, 13, 1979. https://doi.org/10.3390/pharmaceutics13111979
Mabate B, Daub CD, Malgas S, Edkins AL, Pletschke BI. A Combination Approach in Inhibiting Type 2 Diabetes-Related Enzymes Using Ecklonia radiata Fucoidan and Acarbose. Pharmaceutics. 2021; 13(11):1979. https://doi.org/10.3390/pharmaceutics13111979
Chicago/Turabian StyleMabate, Blessing, Chantal Désirée Daub, Samkelo Malgas, Adrienne Lesley Edkins, and Brett Ivan Pletschke. 2021. "A Combination Approach in Inhibiting Type 2 Diabetes-Related Enzymes Using Ecklonia radiata Fucoidan and Acarbose" Pharmaceutics 13, no. 11: 1979. https://doi.org/10.3390/pharmaceutics13111979