Critical Aspects in the Preparation of Extemporaneous Flecainide Acetate Oral Solution for Paediatrics
Abstract
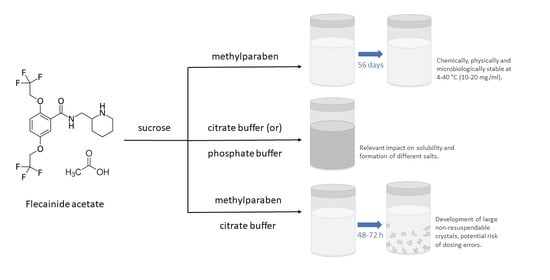
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oral Solutions
2.3. Determination of Solubility
2.4. Chemical and Physical Stability Study
2.5. Precipitate Isolation and Characterisation
2.6. Differential Scanning Calorimetry and Infrared Studies
2.7. Drug Content
2.8. Osmolality Measurement and Microbiological Evaluation
3. Results and Discussion
3.1. Solubility Study
3.2. Characterisation of the Precipitates
3.3. Chemical and Physical Stability Study
3.4. Osmolality and Microbiological Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodieux, F.; Vutskits, L.; Posfay-Barbe, K.M.; Habre, W.; Piguet, V.; Desmeules, J.A.; Samer, C.F. When the Safe Alternative Is Not That Safe: Tramadol Prescribing in Children. Front. Pharmacol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Gore, R.; Chugh, P.K.; Tripathi, C.D.; Lhamo, Y.; Gautam, S. Pediatric Off-Label and Unlicensed Drug Use and Its Implications. Curr. Clin. Pharmacol. 2017, 12, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Musazzi, U.M.; Franceschini, I.; Berti, I.; Paragò, V.; Cardosi, L.; Minghetti, P. Is Propranolol Compounding from Tablet Safe for Pediatric Use? Results from an Experimental Test. Minerva Pediatr. 2014, 66, 355–362. [Google Scholar] [PubMed]
- Council of Europe. European Resolution CM/Res(2016)1 on Quality and Safety Assurance Requirements for Medicinal Products Prepared in Pharmacies for the Special Needs of Patients. Available online: https://search.coe.int/cm/Pages/result_details.aspx?ObjectID=090000168065c132 (accessed on 1 July 2021).
- Norme Di Buona Preparazione Dei Medicinali in Farmacia. In Farmacopea Ufficiale Della Repubblica Italiana, 12th ed.; Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 2008; p. 1415.
- Minghetti, P.; Pantano, D.; Gennari, C.G.M.; Casiraghi, A. Regulatory Framework of Pharmaceutical Compounding and Actual Developments of Legislation in Europe. Health Policy 2014, 117, 328–333. [Google Scholar] [CrossRef]
- Zanon, D.; Selmin, F.; Centin, G.; Maximova, N.; Casiraghi, A.; Minghetti, P. Stability of High Concentrated Triple Intrathecal Therapy for Pediatrics and Mitigation Strategies. Eur. J. Pharm. Sci. 2021, 167, 106039. [Google Scholar] [CrossRef]
- Casiraghi, A.; Musazzi, U.M.; Rocco, P.; Franzè, S.; Minghetti, P. Topical Treatment of Infantile Haemangiomas: A Comparative Study on the Selection of a Semi-Solid Vehicle. Skin Pharmacol. Physiol. 2016, 29, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casiraghi, A.; Gennari, C.G.; Musazzi, U.M.; Ortenzi, M.A.; Bordignon, S.; Minghetti, P. Mucoadhesive Budesonide Formulation for the Treatment of Eosinophilic Esophagitis. Pharmaceutics 2020, 12, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, R.; Stachnik, J.M.; Echizen, H. Clinical Pharmacokinetics of Drugs in Patients with Heart Failure: An Update (Part 2, Drugs Administered Orally). Clin. Pharmacokinet. 2014, 53, 1083–1114. [Google Scholar] [CrossRef]
- Perry, J.C.; Garson, A. Flecainide Acetate for Treatment of Tachyarrhythmias in Children: Review of World Literature on Efficacy, Safety, and Dosing. Am. Heart J. 1992, 124, 1614–1621. [Google Scholar] [CrossRef]
- Cunningham, T.; Uzun, O.; Morris, R.; Franciosi, S.; Wong, A.; Jeremiasen, I.; Sherwin, E.; Sanatani, S. The Safety and Effectiveness of Flecainide in Children in the Current Era. Pediatr. Cardiol. 2017, 38, 1633–1638. [Google Scholar] [CrossRef]
- British National Formulary for Children 2016–2017; Pharmaceutical Press: London, UK, 2016.
- Haywood, A.; Glass, B.D. Liquid Dosage Forms Extemporaneously Prepared from Commercially Available Products—Considering New Evidence on Stability. J. Pharm. Pharm. Sci. 2013, 16, 441–455. [Google Scholar] [CrossRef] [Green Version]
- Santoveña, A.; Charola, I.; Suárez-González, J.; Teigell-Pérez, N.; García-van Nood, S.; Soriano, M.; Fariña, J.B. Development of a Novel Physico-Chemically and Microbiologically Stable Oral Solution of Flecainide for Pediatrics. Pharm. Dev. Technol. 2018, 23, 978–985. [Google Scholar] [CrossRef]
- Wiest, D.B.; Garner, S.S.; Pagacz, L.R.; Zeigler, V. Stability of Flecainide Acetate in an Extemporaneously Compounded Oral Suspension. Am. J. Hosp. Pharm. 1992, 49, 1467–1470. [Google Scholar] [CrossRef]
- Allen, L.V.J.; Erickson, M.A., 3rd. Stability of Baclofen, Captopril, Diltiazem Hydrochloride, Dipyridamole, and Flecainide Acetate in Extemporaneously Compounded Oral Liquids. AJHP Off. J. Am. Soc. Health Pharm. 1996, 53, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Uriel, M.; Gómez-Rincón, C.; Marro, D. Stability of Regularly Prescribed Oral Liquids Formulated with SyrSpend(®) SF. Pharmazie 2018, 73, 196–201. [Google Scholar] [CrossRef]
- Stuart, A.G.; Wren, C.; Bain, H.H. Is There a Genetic Factor in Flecainide Toxicity? Br. Med. J. 1989, 298, 117–118. [Google Scholar] [CrossRef] [Green Version]
- Helin-Tanninen, M.; Autio, K.; Keski-Rahkonen, P.; Naaranlahti, T.; Järvinen, K. Comparison of Six Different Suspension Vehicles in Compounding of Oral Extemporaneous Nifedipine Suspension for Paediatric Patients. Eur. J. Hosp. Pharm. Sci. Pract. 2012, 19, 432–437. [Google Scholar] [CrossRef]
- El-Ragehy, N.A.; Hassan, N.Y.; Tantawy, M.A.; Abdelkawy, M. Stability-Indicating Chromatographic Methods for Determination of Flecainide Acetate in the Presence of Its Degradation Products; Isolation and Identification of Two of Its Impurities. Biomed. Chromatogr. 2016, 30, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 9th ed.; Physical and Physicochemical Methods (2.2.); Test for Specified Micro-Organisms (2.6.13.); Microbiological Quality of Non-Sterile Pharmaceutical Preparations and Substances for Pharmaceutical Use (5.1.4.); Council Of Europe: Strasbourg, France, 2017.
- Flecainide. Available online: https://hmdb.ca/metabolites/HMDB0015326 (accessed on 12 July 2021).
- Flecainide. Available online: https://go.drugbank.com/drugs/DB01195 (accessed on 31 March 2021).
- Woods, D.J. Flecainide Acetate. Available online: http://www.fshealth.gov.za/subsites/DWoods/Mixtures/flecainide.html (accessed on 31 March 2021).
- SA Health. South Australian Neonatal Medication Guidelines—Flecainide. Available online: https://www.sahealth.sa.gov.au/wps/wcm/connect/dcc5f7d4-7c6e-46b5-a6a7-6cd7f249e7da/Flecainide_Neo_v1_0.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-dcc5f7d4-7c6e-46b5-a6a7-6cd7f249e7da-nGtHHWO (accessed on 1 April 2021).
- Tessarolo Silva, F.; Pedreira, G.C.; Medeiros, S.A.; Bortolotto, A.L.; Araujo Silva, B.; Hurrey, M.; Madhavapeddi, P.; Schuler, C.; Belardinelli, L.; Verrier, R.L. Multimodal Mechanisms and Enhanced Efficiency of Atrial Fibrillation Cardioversion by Pulmonary Delivery of a Novel Flecainide Formulation. J. Cardiovasc. Electrophysiol. 2020, 31, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef] [Green Version]
- Santoveña-Estévez, A.; Suárez-González, J.; Vera, M.; González-Martín, C.; Soriano, M.; Fariña, J.B. Effectiveness of Antimicrobial Preservation of Extemporaneous Diluted Simple Syrup Vehicles for Pediatrics. J. Pediatr. Pharmacol. Ther. 2018, 23, 405–409. [Google Scholar] [CrossRef]
- Ghulam, A.; Keen, K.; Tuleu, C.; Wong, I.C.-K.; Long, P.F. Poor Preservation Efficacy versus Quality and Safety of Pediatric Extemporaneous Liquids. Ann. Pharmacother. 2007, 41, 857–860. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Reflection Paper on the Use of Methyl- and Propylparaben as Excipients in Human Medicinal Products for Oral Use; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Yellepeddi, V.; Vangara, K. Excipients in Pediatric Formulations: Biopharmaceutical and Toxicological Considerations. In Excipient Applications in Formulation Design and Drug Delivery; Springer: Cham, Switzerland, 2015; pp. 497–519. [Google Scholar] [CrossRef]
- Flecainide—Substance Detail, Predicted Properties (Calculated using Advanced Chemistry Development Software V11.02, © 1994–2021 ACD/Labs). Available online: https://scifinder-n.cas.org/searchDetail/substance/6133810cb914160d7af8bb26/substanceDetails (accessed on 4 September 2021).
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, M.; Branton, A.; Trivedi, D.; Nayak, G.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment. J. Chromatogr. Sep. Tech. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, S.D.; Kulandaivelu, R.; Tsn, S.N.; Lee, M.H. A Facile Electrochemical Approach for the Deposition of Iron-Manganese Phosphate Composite Coatings on Aluminium. RSC Adv. 2014, 5, 988–1008. [Google Scholar] [CrossRef]
- Carella, F.; Degli Esposti, L.; Barreca, D.; Rizzi, G.A.; Martra, G.; Ivanchenko, P.; Escolano Casado, G.; Gomez Morales, J.; Delgado Lòpez, J.M.; Tampieri, A.; et al. Role of Citrate in the Formation of Enamel-like Calcium Phosphate Oriented Nanorod Arrays. CrystEngComm 2019, 21, 4684–4689. [Google Scholar] [CrossRef]
- Barness, L.A.; Mauer, A.M.; Holliday, M.A.; Anderson, A.S.; Dallman, P.R.; Forbes, G.B.; Goldbloom, R.B.; Haworth, J.C.; Jesse, M.J.; Scriver, C.R.; et al. Commentary on Breast-Feeding and Infant Formulas, Including Proposed Standards for Formulas. Pediatrics 1976, 57, 278–285. [Google Scholar]
- Shah, D.D.; Kuzmov, A.; Clausen, D.; Siu, A.; Robinson, C.A.; Kimler, K.; Meyers, R.; Shah, P. Osmolality of Commonly Used Oral Medications in the Neonatal Intensive Care Unit. J. Pediatr. Pharmacol. Ther. JPPT Off. J. PPAG 2021, 26, 172–178. [Google Scholar] [CrossRef]
- Nowak, K.; Ratajczak-Wrona, W.; Górska, M.; Jabłońska, E. Parabens and Their Effects on the Endocrine System. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Excipients in the Dossier for Application for Marketing Authorisation of a Medicinal Product; European Medicines Agency: Amsterdam, The Netherlands, 2007. [Google Scholar]





| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
|---|---|---|---|---|---|---|---|---|
| Sucrose | 20 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Methylparaben | - | - | 0.07 | 0.07 | - | 0.07 | - | 0.07 |
| Glycerol | - | - | - | 10 | - | - | - | - |
| Citric acid | - | - | - | - | 0.1 | 0.1 | - | - |
| Sodium citrate | - | - | - | - | 0.08 | 0.08 | - | - |
| Monosodium phosphate | - | - | - | - | - | - | 0.21 | 0.21 |
| Sodium hydroxide (1M) * | - | - | - | - | - | - | q.s. | q.s. |
| Water ** | 87.5 | 74.5 | 74.5 | 66.5 | 74.5 | 74.5 | 74.5 | 74.5 |
| Storage Temperature | Actual Initial Concentration (mg/mL) | % Labeled Concentration Remaining | |||
|---|---|---|---|---|---|
| 14 Days | 28 Days | 42 Days | 56 Days | ||
| 10 mg/mL flecainide acetate oral solution | |||||
| 4 °C | 10.3 ± 0.0 | 102 ± 2 | 102 ± 1 | 101 ± 3 | 100 ± 1 |
| 25 °C | 104 ± 1 | 103 ± 2 | 100 ± 1 | 100 ± 2 | |
| 40 °C | 103 ± 2 | 104 ± 1 | 102 ± 4 | 103 ± 4 | |
| 20 mg/mL flecainide acetate oral solution | |||||
| 4 °C | 20.1 ± 0.0 | 102 ± 2 | 102 ± 3 | 98 ± 1 | 101 ± 1 |
| 25 °C | 102 ± 1 | 101 ± 2 | 99 ± 1 | 100 ± 1 | |
| 40 °C | 102 ± 1 | 102 ± 2 | 98 ± 1 | 102 ± 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiraghi, A.; Centin, G.; Selmin, F.; Picozzi, C.; Minghetti, P.; Zanon, D. Critical Aspects in the Preparation of Extemporaneous Flecainide Acetate Oral Solution for Paediatrics. Pharmaceutics 2021, 13, 1963. https://doi.org/10.3390/pharmaceutics13111963
Casiraghi A, Centin G, Selmin F, Picozzi C, Minghetti P, Zanon D. Critical Aspects in the Preparation of Extemporaneous Flecainide Acetate Oral Solution for Paediatrics. Pharmaceutics. 2021; 13(11):1963. https://doi.org/10.3390/pharmaceutics13111963
Chicago/Turabian StyleCasiraghi, Antonella, Giorgio Centin, Francesca Selmin, Claudia Picozzi, Paola Minghetti, and Davide Zanon. 2021. "Critical Aspects in the Preparation of Extemporaneous Flecainide Acetate Oral Solution for Paediatrics" Pharmaceutics 13, no. 11: 1963. https://doi.org/10.3390/pharmaceutics13111963