Sorafenib Repurposing for Ophthalmic Delivery by Lipid Nanoparticles: A Preliminary Study
Abstract
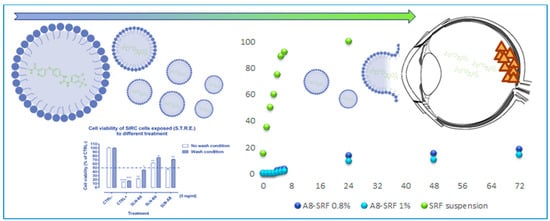
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticles Preparation
2.3. Photon Correlation Spectroscopy (PCS)
2.4. Sterilisation by Filtration
2.5. Osmolality and pH
2.6. Turbiscan® AG Station
2.7. Cell Viability Studies
2.8. Encapsulation Efficiency and In Vitro Drug Release
2.9. High-Performance Liquid Chromatography (HPLC) Analyses
2.10. Stability and Interaction of Nanoparticles in the Presence of Ocular Mucus Component
2.10.1. Physico-Chemical Evaluation
2.10.2. Mucoadhesive Strength
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterisation
3.2. In Vitro Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buzzacco, D.M.; Abdel-Rahman, M.H.; Park, S.; Davidorf, F.; Olencki, T.; Cebulla, C.M. Long-Term Survivors with Metastatic Uveal Melanoma. Open. Ophthalmol. J. 2012, 6, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederkorn, A.; Wackernagel, W.; Artl, M.; Schwantzer, G.; Aigner, B.; Richtig, E. Response of Patients with Metastatic Uveal Melanoma to Combined Treatment with Fotemustine and Sorafenib. Acta Ophthalmol. 2014, 92, e696–e697. [Google Scholar] [CrossRef] [PubMed]
- Velho, T.R.; Kapiteijn, E.; Jager, M.J. New Therapeutic Agents in Uveal Melanoma. Anticancer Res. 2012, 32, 2591–2598. [Google Scholar] [PubMed]
- Collaborative Ocular Melanoma Study Group. Assessment of Metastatic Disease Status at Death in 435 Patients with Large Choroidal Melanoma in the Collaborative Ocular Melanoma Study (Coms): Coms Report No. 15. Arch. Ophthalmol. 2001, 119, 670–676. [Google Scholar] [CrossRef]
- Kujala, E.; Makitie, T.; Kivela, T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [Green Version]
- Augsburger, J.J.; Correa, Z.M.; Shaikh, A.H. Effectiveness of Treatments for Metastatic Uveal Melanoma. Am. J. Ophthalmol. 2009, 148, 119–127. [Google Scholar] [CrossRef]
- Bhatia, S.; Moon, J.; Margolin, K.A.; Weber, J.S.; Lao, C.D.; Othus, M.; Aparicio, A.M.; Ribas, A.; Sondak, V.K. Phase Ii Trial of Sorafenib in Combination with Carboplatin and Paclitaxel in Patients with Metastatic Uveal Melanoma: Swog S0512. PLoS ONE 2012, 7, e48787. [Google Scholar] [CrossRef]
- Mouriaux, F.; Servois, V.; Parienti, J.J.; Lesimple, T.; Thyss, A.; Dutriaux, C.; Neidhart-Berard, E.M.; Penel, N.; Delcambre, C.; Peyro Saint Paul, L.; et al. Sorafenib in Metastatic Uveal Melanoma: Efficacy, Toxicity and Health-Related Quality of Life in a Multicentre Phase Ii Study. Br. J. Cancer 2016, 115, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Benizri, S.; Ferey, L.; Alies, B.; Mebarek, N.; Vacher, G.; Appavoo, A.; Staedel, C.; Gaudin, K.; Barthelemy, P. Nucleoside-Lipid-Based Nanocarriers for Sorafenib Delivery. Nanoscale Res. Lett. 2018, 13, 17. [Google Scholar] [CrossRef]
- Bondi, M.L.; Botto, C.; Amore, E.; Emma, M.R.; Augello, G.; Craparo, E.F.; Cervello, M. Lipid Nanocarriers Containing Sorafenib Inhibit Colonies Formation in Human Hepatocarcinoma Cells. Int. J. Pharm. 2015, 493, 75–85. [Google Scholar] [CrossRef]
- Santonocito, M.; Zappulla, C.; Viola, S.; La Rosa, L.R.; Solfato, E.; Abbate, I.; Tarallo, V.; Apicella, I.; Platania, C.B.M.; Maugeri, G.; et al. Assessment of a New Nanostructured Microemulsion System for Ocular Delivery of Sorafenib to Posterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 4404. [Google Scholar] [CrossRef]
- Mangana, J.; Levesque, M.P.; Karpova, M.B.; Dummer, R. Sorafenib in Melanoma. Expert Opin. Investig. Drugs 2012, 21, 557–568. [Google Scholar] [CrossRef]
- Grillone, A.; Riva, E.R.; Mondini, A.; Forte, C.; Calucci, L.; Innocenti, C.; de Julian Fernandez, C.; Cappello, V.; Gemmi, M.; Moscato, S.; et al. Active Targeting of Sorafenib: Preparation, Characterization, and in Vitro Testing of Drug-Loaded Magnetic Solid Lipid Nanoparticles. Adv. Healthc. Mater. 2015, 4, 1681–1690. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F.M.; Bianchera, A.; Gasco, P.; Nicoli, S.; Pescina, S. Lipid-Based Nanocarriers for Ophthalmic Administration: Towards Experimental Design Implementation. Pharmaceutics 2021, 13, 447. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; Garcia, M.L. Lipid Nanoparticles (Sln, Nlc): Overcoming the Anatomical and Physiological Barriers of the Eye—Part I—Barriers and Determining Factors in Ocular Delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef]
- Szilágyi, B.Á.; Mammadova, A.; Gyarmati, B.; Szilágyi, A. Mucoadhesive Interactions between Synthetic Polyaspartamides and Porcine Gastric Mucin on the Colloid Size Scale. Colloids Surf. B Biointerfaces 2020, 194, 111219. [Google Scholar] [CrossRef]
- Carbone, C.; Fuochi, V.; Zielinska, A.; Musumeci, T.; Souto, E.B.; Bonaccorso, A.; Puglia, C.; Petronio Petronio, G.; Furneri, P.M. Dual-Drugs Delivery in Solid Lipid Nanoparticles for the Treatment of Candida Albicans Mycosis. Colloids Surf. B Biointerfaces 2020, 186, 110705. [Google Scholar] [CrossRef]
- Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-Nlc with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials 2020, 10, 898. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Musumeci, T.; Carbone, C.; Vicari, L.; Lauro, M.R.; Puglisi, G. Revisiting the Role of Sucrose in Plga-Peg Nanocarrier for Potential Intranasal Delivery. Pharm. Dev. Technol. 2018, 23, 265–274. [Google Scholar] [CrossRef]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernandez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, Biocompatibility and Antioxidant Activity of Peg-Modified Liposomes Containing Resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-Based Strategies for Treatment of Ocular Disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, E.; Ozeki, H.; Kunou, N.; Ogura, Y. Effect of Particle Size of Polymeric Nanospheres on Intravitreal Kinetics. Ophthalmic. Res. 2001, 33, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Offerta, A.; Carbone, C.; Bonina, F.; Pignatello, R.; Puglisi, G. Lipid Nanocarriers (Lnc) and Their Applications in Ocular Drug Delivery. Curr. Med. Chem. 2015, 22, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Doktorovova, S.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L. Feasibility of Lipid Nanoparticles for Ocular Delivery of Anti-Inflammatory Drugs. Curr. Eye Res. 2010, 35, 537–552. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kwon, J.; Shin, S.; Eun, Y.G.; Shin, J.H.; Lee, G.J. Optimization of Saliva Collection and Immunochromatographic Detection of Salivary Pepsin for Point-of-Care Testing of Laryngopharyngeal Reflux. Sensors 2020, 20, 325. [Google Scholar] [CrossRef] [Green Version]
- Kotsilkova, R.; Borovanska, I.; Todorov, P.; Ivanov, E.; Menseidov, D.; Chakraborty, S.; Bhattacharjee, C. Tensile and Surface Mechanical Properties of Polyethersulphone (Pes) and Polyvinylidene Fluoride (Pvdf) Membranes. J. Theor. Appl. Mech. Bulg. 2018, 48, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.H.; Jang, J.H.; Cho, H.Y.; Lee, Y.B. Soft- and Hard-Lipid Nanoparticles: A Novel Approach to Lymphatic Drug Delivery. Arch. Pharm. Res. 2018, 41, 797–814. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Iwasawa, A.; Ayaki, M.; Niwano, Y. Cell viability score (CVS) as a good indicator of critical concentration of ben-zalkonium chloride for toxicity in cultured ocular surface cell lines. Regul. Toxicol. Pharmacol. 2013, 66, 177–183. [Google Scholar] [CrossRef]
- Freeman, P.D.; Kahook, M.Y. Preservatives in Topical Ophthalmic Medications: Historical and Clinical Perspectives. Exp. Rev. Ophthalmol. 2009, 4, 59–64. [Google Scholar] [CrossRef]
- Mikkelson, T.J.; Chrai, S.S.; Robinson, J.R. Altered Bioavailability of Drugs in the Eye Due to Drug-Protein Interaction. J. Pharm. Sci. 1973, 62, 1648–1653. [Google Scholar] [CrossRef]
- EURL ECVAM Database on Alternative Methods to Animal Experimentation. Available online: http://cidportal.jrc.ec.europa.eu/ftp/jrc-opendata/EURL-ECVAM/datasets/DBALM/LATEST/online/DBALM_docs/17_P_MTT%20Assay.pdf (accessed on 5 October 2021).
- Takahashi, Y.; Hayashi, K.; Abo, T.; Koike, M.; Sakaguchi, H.; Nishiyama, N. The Short Time Exposure (STE) test for predicting eye irritation potential: Intra-laboratory reproducibility and correspondence to globally harmonized system (GHS) and EU eye irritation classification for 109 chemicals. Toxicol. Vitr. 2011, 25, 1425–1434. [Google Scholar] [CrossRef]
- Carbone, C.; Campisi, A.; Manno, D.; Serra, A.; Spatuzza, M.; Musumeci, T.; Bonfanti, R.; Puglisi, G. The Critical Role of Didodecyldimethylammonium Bromide on Physico-Chemical, Technological and Biological Properties of Nlc. Colloids Surf. B Biointerfaces 2014, 121, 1–10. [Google Scholar] [CrossRef]
- Carbone, C.; Tomasello, B.; Ruozi, B.; Renis, M.; Puglisi, G. Preparation and Optimization of Pit Solid Lipid Nanoparticles Via Statistical Factorial Design. Eur. J. Med. Chem. 2012, 49, 110–117. [Google Scholar] [CrossRef]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.; Sanchez, A.; Alonso, M.J. Chitosan Nanoparticles as New Ocular Drug Delivery Systems: In Vitro Stability, In Vivo Fate, and Cellular Toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef]
- Tobio, M.; Sanchez, A.; Vila, A.; Soriano, I.I.; Evora, C.; Vila-Jato, J.L.; Alonso, M.J. The Role of Peg on the Stability in Digestive Fluids and in Vivo Fate of Peg-Pla Nanoparticles Following Oral Administration. Colloids Surf. B Biointerfaces 2000, 18, 315–323. [Google Scholar] [CrossRef]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Effect of Lysozyme on the Stability of Polyester Nanocapsules and Nanoparticles: Stabilization Approaches. Biomaterials 1997, 18, 1305–1310. [Google Scholar] [CrossRef]
- Mendes, A.C.; Sevilla Moreno, J.; Hanif, M.; Douglas, T.E.L.; Chen, M.; Chronakis, I.S. Morphological, Mechanical and Mucoadhesive Properties of Electrospun Chitosan/Phospholipid Hybrid Nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. [Google Scholar] [CrossRef] [Green Version]
- Alp, G.; Aydogan, N. Lipid-Based Mucus Penetrating Nanoparticles and Their Biophysical Interactions with Pulmonary Mucus Layer. Eur. J. Pharm. Biopharm. 2020, 149, 45–57. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Cimino, C.; Manno, D.E.; Tomasello, B.; Serra, A.; Musumeci, T.; Puglisi, G.; Pignatello, R.; Carbone, C. Essential oils loaded NLC for potential intranasal administration. Pharmaceutics 2021, 13, 1166. [Google Scholar] [CrossRef]
- Cordeiro, S.S.B.; Martins, A.M.; Ribeiro, H.M.; Gonçalves, L.; Marto, J. Antioxidant-Loaded Mucoadhesive Nanoparticles for Eye Drug Delivery: A New Strategy to Reduce Oxidative Stress. Processes 2021, 9, 379. [Google Scholar] [CrossRef]
- Kubackova, J.; Holas, O.; Zbytovska, J.; Vranikova, B.; Zeng, G.; Pavek, P.; Mullertz, A. Oligonucleotide Delivery across the Caco-2 Monolayer: The Design and Evaluation of Self-Emulsifying Drug Delivery Systems (Sedds). Pharmaceutics 2021, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.B.; Long, J.; Zhao, Y.Y.; Yang, J.B.; Jiang, W.; Liu, Q.Z.; Yan, K.; Li, L.; Wang, Y.C.; Lian, Z.X. Adaptive Immune Cells Are Necessary for the Enhanced Therapeutic Effect of Sorafenib-Loaded Nanoparticles. Biomater. Sci. 2018, 6, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.K.; Sharmila, P.; Bardia, A.; Chandrakala, L.; Raju, N.; Sravani, G.; Sastry, B.V.S.; Habeeb, M.A.; Khan, A.A.; Dhayal, M. Use of Biocompatible Sorafenib-Gold Nanoconjugates for Reversal of Drug Resistance in Human Hepatoblatoma Cells. Sci. Rep. 2017, 7, 8539. [Google Scholar] [CrossRef] [Green Version]
- Tatsugami, K.; Oya, M.; Kabu, K.; Akaza, H. Evaluation of Efficacy and Safety of Sorafenib in Kidney Cancer Patients Aged 75 Years and Older: A Propensity Score-Matched Analysis. Br. J. Cancer 2018, 119, 241–247. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, J.; Zhou, W.; Huang, X.F.; Chen, Q.; Wang, W.; Zhai, L.; Li, S.; Tang, Z. Sorafenib Blocks the Activation of the Hif-2alpha/Vegfa/Epha2 Pathway, and Inhibits the Rapid Growth of Residual Liver Cancer Following High-Intensity Focused Ultrasound Therapy In Vivo. Pathol. Res. Pract. 2021, 220, 153270. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, L.; Jin, H.; Chen, Y.; Cheng, D.; Jiang, Y. Sorafenib-Loaded Long-Circulating Nanoliposomes for Liver Cancer Therapy. Biomed. Res. Int. 2020, 2020, 1351046. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, M.A.; de la Cruz-Ojeda, P.; Gallego, P.; Navarro-Villaran, E.; Stankova, P.; Del Campo, J.A.; Kucera, O.; Elkalaf, M.; Maseko, T.E.; Cervinkova, Z.; et al. Dose-Dependent Regulation of Mitochondrial Function and Cell Death Pathway by Sorafenib in Liver Cancer Cells. Biochem. Pharmacol. 2020, 176, 113902. [Google Scholar] [CrossRef]
- Ebadi, M.; Bullo, S.; Buskara, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Release of a Liver Anticancer Drug, Sorafenib from Its Pva/Ldh- and Peg/Ldh-Coated Iron Oxide Nanoparticles for Drug Delivery Applications. Sci. Rep. 2020, 10, 21521. [Google Scholar] [CrossRef]
- Kernt, M.; Neubauer, A.S.; Liegl, R.G.; Hirneiss, C.; Alge, C.S.; Wolf, A.; Ulbig, M.W.; Kampik, A. Sorafenib Prevents Human Retinal Pigment Epithelium Cells from Light-Induced Overexpression of Vegf, Pdgf and Plgf. Br. J. Ophthalmol. 2010, 94, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Kernt, M.; Liegl, R.G.; Rueping, J.; Neubauer, A.S.; Haritoglou, C.; Lackerbauer, C.A.; Eibl, K.H.; Ulbig, M.W.; Kampik, A. Sorafenib Protects Human Optic Nerve Head Astrocytes from Light-Induced Overexpression of Vascular Endothelial Growth Factor, Platelet-Derived Growth Factor, and Placenta Growth Factor. Growth Factors 2010, 28, 211–220. [Google Scholar] [CrossRef]
- Kernt, M.; Staehler, M.; Stief, C.; Kampik, A.; Neubauer, A.S. Resolution of Macular Oedema in Occult Choroidal Neovascularization under Oral Sorafenib Treatment. Acta. Ophthalmol. 2008, 86, 456–458. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Li, W.; Correia, A.; Khan, M.M.; Rahim, M.A.; Santos, H.A. Microfluidic Fabrication and Characterization of Sorafenib-Loaded Lipid-Polymer Hybrid Nanoparticles for Controlled Drug Delivery. Int. J. Pharm. 2020, 581, 119275. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, X.; Lv, Y.; Zhao, Y. Low Density Lipoprotein Receptor (Ldlr)-Targeted Lipid Nanoparticles for the Delivery of Sorafenib and Dihydroartemisinin in Liver Cancers. Life Sci. 2019, 239, 117013. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Yang, W.; Yu, M.; Sun, S.; Xie, B. Improved Oral Bioavailability and Liver Targeting of Sorafenib Solid Lipid Nanoparticles in Rats. AAPS Pharm. Sci. Tech. 2018, 19, 761–768. [Google Scholar] [CrossRef]
- Herranz-Blanco, B.; Arriaga, L.R.; Makila, E.; Correia, A.; Shrestha, N.; Mirza, S.; Weitz, D.A.; Salonen, J.; Hirvonen, J.; Santos, H.A. Microfluidic Assembly of Multistage Porous Silicon-Lipid Vesicles for Controlled Drug Release. Lab Chip 2014, 14, 1083–1086. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, W.; Sorenson, C.M.; Sheibani, N.; Albert, D.M.; Senanayake, T.; Vinogradov, S.; Henkin, J.; Zhang, H.F. Sustaining Intravitreal Residence with L-Arginine Peptide-Conjugated Nanocarriers. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5142–5150. [Google Scholar] [CrossRef]
- Fu, T.; Yi, J.; Lv, S.; Zhang, B. Ocular Amphotericin B Delivery by Chitosan-Modified Nanostructured Lipid Carriers for Fungal Keratitis-Targeted Therapy. J. Liposome Res. 2017, 27, 228–233. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R. Lipid Nanocarrier: An Efficient Approach Towards Ocular Delivery of Hydrophilic Drug (Valacyclovir). AAPS Pharm. Sci. Tech. 2017, 18, 884–894. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, C.; Wang, Y.; Song, C. Pharmacokinetics and Tolerance Study of Intravitreal Injection of Dexamethasone-Loaded Nanoparticles in Rabbits. Int. J. Nanomed. 2009, 4, 175–183. [Google Scholar] [CrossRef] [Green Version]






| Filtration | SLN | Z-Ave ± S.D. (nm) | PDI ± S.D. |
|---|---|---|---|
| Not filtered | A8 | 96.63 ± 2.05 | 0.177 ± 0.020 |
| B8 | 70.67 ± 2.08 | 0.126 ± 0.003 | |
| B9 | 123.5 ± 1.36 | 0.139 ± 0.012 | |
| Filtered (PVDF) | A8 | 95.53 ± 0.63 | 0.197 ± 0.080 |
| B8 | 72.03 ± 0.46 | 0.178 ± 0.008 * | |
| B9 | 117.18 ± 0.82 | 0.165 ± 0.020 |
| SLN | Z-Ave ± S.D. (nm) | PDI ± S.D. | pH ± S.D. | Osmolality (mOsm/Kg) ± S.D. | EE% ± S.D. |
|---|---|---|---|---|---|
| A8-SRF 0.8% | 127.85 ± 1.50 | 0.215 ± 0.014 | 6.33 ± 0.85 | 308 ± 2 | 75.0 ± 2.1 |
| A8-SRF 1% | 150.12 ± 1.85 | 0.180 ± 0.013 | 6.25 ± 0.72 | 302 ± 3 | 74.5 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaccorso, A.; Pepe, V.; Zappulla, C.; Cimino, C.; Pricoco, A.; Puglisi, G.; Giuliano, F.; Pignatello, R.; Carbone, C. Sorafenib Repurposing for Ophthalmic Delivery by Lipid Nanoparticles: A Preliminary Study. Pharmaceutics 2021, 13, 1956. https://doi.org/10.3390/pharmaceutics13111956
Bonaccorso A, Pepe V, Zappulla C, Cimino C, Pricoco A, Puglisi G, Giuliano F, Pignatello R, Carbone C. Sorafenib Repurposing for Ophthalmic Delivery by Lipid Nanoparticles: A Preliminary Study. Pharmaceutics. 2021; 13(11):1956. https://doi.org/10.3390/pharmaceutics13111956
Chicago/Turabian StyleBonaccorso, Angela, Veronica Pepe, Cristina Zappulla, Cinzia Cimino, Angelo Pricoco, Giovanni Puglisi, Francesco Giuliano, Rosario Pignatello, and Claudia Carbone. 2021. "Sorafenib Repurposing for Ophthalmic Delivery by Lipid Nanoparticles: A Preliminary Study" Pharmaceutics 13, no. 11: 1956. https://doi.org/10.3390/pharmaceutics13111956