Retinoic Acid-Containing Liposomes for the Induction of Antigen-Specific Regulatory T Cells as a Treatment for Autoimmune Diseases
Abstract
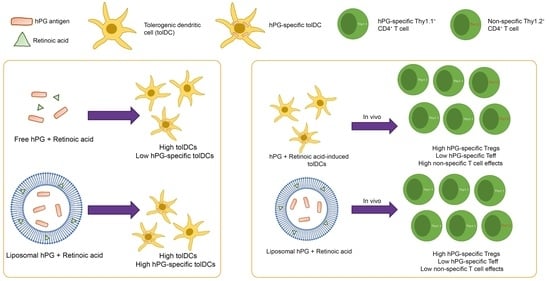
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Peptides and Conjugates
2.2. Liposome Preparation
2.3. Liposome Characterization
2.4. Mice
2.5. Bone Marrow-Derived DC (BMDC) Culture
2.6. T Cell Isolation and Co-Culture with BMDCs
2.7. Adoptive Transfer of hPG TCR-Specific T Cells
2.8. Flow Cytometry
2.9. Live Cell Imaging
3. Results
3.1. RA Induces a tolDC Phenotype in BMDCs In Vitro
3.2. RA Can Be Efficiently Encapsulated into Liposomes and Retains tolDC Inducing Effects
3.3. Liposomal Co-Delivery of hPG and RA Leads to Aantigen-Associated tolDC Induction In Vitro
3.4. tolDCs Generated with hPG/RA Liposomes Skew T Cells towards a Regulatory Phenotype In Vitro
3.5. hPG and RA Delivered by tolDCs, Liposomes or Free Affect Splenic CD11c+ Cell Populations In Vivo
3.6. hPG and RA Delivered by tolDCs, Liposomes or Free Affect Splenic CD4+ T Cell Populations In Vivo
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willame, C.; Dodd, C.; van der Aa, L.; Picelli, G.; Emborg, H.-D.; Kahlert, J.; Gini, R.; Huerta, C.; Martín-Merino, E.; McGee, C.; et al. Incidence Rates of Autoimmune Diseases in European Healthcare Databases: A Contribution of the ADVANCE Project. Drug Saf. 2021, 44, 383–395. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Audiger, C.; Rahman, M.J.; Yun, T.J.; Tarbell, K.V.; Lesage, S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J. Immunol. 2017, 198, 2223–2231. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Ming, Y.; Yang, C. Regulatory B cells: The cutting edge of immune tolerance in kidney transplantation. Cell Death Dis. 2018, 9, 109. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Riedhammer, C.; Weissert, R. Antigen Presentation, Autoantigens, and Immune Regulation in Multiple Sclerosis and Other Autoimmune Diseases. Front. Immunol. 2015, 6, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padovan, E.; Landmann, R.M.; De Libero, G. How pattern recognition receptor triggering influences T cell responses: A new look into the system. Trends Immunol. 2007, 28, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 2001, 13, 45–51. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.E.; Swan, D.J.; Sayers, B.L.; Harry, R.A.; Patterson, A.M.; Von Delwig, A.; Robinson, J.H.; Isaacs, J.D.; Hilkens, C.M.U. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J. Leukoc. Biol. 2008, 85, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Jansen, M.A.A.; Spiering, R.; Ludwig, I.S.; Van Eden, W.; Hilkens, C.M.U.; Broere, F. Matured Tolerogenic Dendritic Cells Effectively Inhibit Autoantigen Specific CD4+ T Cells in a Murine Arthritis Model. Front. Immunol. 2019, 10, 2068. [Google Scholar] [CrossRef]
- Van Eden, W.; Jansen, M.A.A.; De Wolf, A.C.M.; Ludwig, I.S.; Leufkens, P.; Broere, F. The Immunomodulatory Potential of tolDCs Loaded with Heat Shock Proteins. Front. Immunol. 2017, 8, 1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-González, P.; Ubilla-Olguín, G.; Catalán, D.; Schinnerling, K.; Aguillón, J.C. Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun. Rev. 2016, 15, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Qin, J.; Bennett, C.; Qian, S.; Fung, J.; Hamilton, T.A.; Lu, L. All-transRetinoic Acid Induces Arginase-1 and Inducible Nitric Oxide Synthase–Producing Dendritic Cells with T Cell Inhibitory Function. J. Immunol. 2014, 192, 5098–5108. [Google Scholar] [CrossRef] [Green Version]
- Koprivica, I.; Gajić, D.; Saksida, T.; Cavalli, E.; Auci, D.; Despotović, S.; Pejnović, N.; Stošić-Grujičić, S.; Nicoletti, F. Orally delivered all-trans-retinoic acid- and transforming growth factor-beta-loaded microparticles ameliorate type 1 diabetes in mice. Eur. J. Pharmacol. 2019, 864, 172721. [Google Scholar] [CrossRef]
- Iwata, M.; Yokota, A. Retinoic Acid Production by Intestinal Dendritic Cells. Vitam. Horm. 2011, 86, 127–152. [Google Scholar] [CrossRef]
- Theodosiou, M.; Laudet, V.; Schubert, M. From carrot to clinic: An overview of the retinoic acid signaling pathway. Cell Mol. Life Sci. 2010, 67, 1423–1445. [Google Scholar] [CrossRef]
- Benson, M.J.; Pino-Lagos, K.; Rosemblatt, M.; Noelle, R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007, 204, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.-M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coombes, J.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.-M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Suuring, M.; Moreau, A. Regulatory Macrophages and Tolerogenic Dendritic Cells in Myeloid Regulatory Cell-Based Therapies. Int. J. Mol. Sci. 2021, 22, 7970. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.T.; Foged, C.; Korsholm, K.S.; Rades, T.; Christensen, D. Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Im-munostimulators. Pharmaceutics 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef]
- Benne, N.; van Duijn, J.; Vigario, F.L.; Leboux, R.; van Veelen, P.; Kuiper, J.; Jiskoot, W.; Slütter, B. Anionic 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) liposomes induce antigen-specific regulatory T cells and prevent atherosclerosis in mice. J. Control. Release 2018, 291, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Angum, F.; Khan, T.; Kaler, J.; Siddiqui, L.; Hussain, A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus 2020, 12, e8094. [Google Scholar] [CrossRef]
- Berlo, S.E.; van Kooten, P.J.; Brink, C.B.T.; Hauet-Broere, F.; Oosterwegel, M.A.; Glant, T.T.; Van Eden, W.; Broeren, C.P. Naive transgenic T cells expressing cartilage proteoglycan-specific TCR induce arthritis upon in vivo activation. J. Autoimmun. 2005, 25, 172–180. [Google Scholar] [CrossRef]
- Reddy, M.; Eirikis, E.; Davis, C.; Davis, H.M.; Prabhakar, U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: An in vitro model to monitor cellular immune function. J. Immunol. Methods 2004, 293, 127–142. [Google Scholar] [CrossRef]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Braun, A.; Worbs, T.; Moschovakis, G.L.; Halle, S.; Hoffmann, K.; Bölter, J.; Münk, A.; Forster, R. Afferent lymph–derived T cells and DCs use different chemokine receptor CCR7–dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 2011, 12, 879–887. [Google Scholar] [CrossRef]
- Ueda, H.; Howson, J.M.; Esposito, L.; Heward, J.; Chamberlain, G.; Rainbow, D.B.; Hunter, K.M.; Smith, A.N.; Di Genova, G.; Herr, M. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003, 423, 506–511. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.-X.; Chen, Y.; Xu, H.; Fitzgerald, D.C.; Zhao, Z.; Rostami, A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental auto-immune encephalomyelitis. J. Immunol. 2008, 181, 2483–2493. [Google Scholar] [CrossRef] [Green Version]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.-W.; Park, C.G.; et al. Differential Antigen Processing by Dendritic Cell Subsets in Vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef]
- Belz, G.T.; Behrens, G.M.; Smith, C.M.; Miller, J.F.; Jones, C.; Lejon, K.; Fathman, C.G.; Mueller, S.; Shortman, K.; Carbone, F.R.; et al. The CD8α+ Dendritic Cell Is Responsible for Inducing Peripheral Self-Tolerance to Tissue-associated Antigens. J. Exp. Med. 2002, 196, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilsborough, J.; George, T.C.; Norment, A.; Viney, J.L. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology 2003, 108, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.O.; Min, S.-Y.; Cho, M.-L.; Park, M.-J.; Jeon, J.-Y.; Lee, J.-S.; Oh, H.-J.; Kang, C.-M.; Park, H.-S.; Park, K.-S.; et al. CD8alpha+ dendritic cells enhance the antigen-specific CD4+ T-cell response and accelerate development of colla-gen-induced arthritis. Immunol. Lett. 2007, 111, 76–83. [Google Scholar] [CrossRef]
- Benne, N.; Leboux, R.J.; Glandrup, M.; van Duijn, J.; Vigario, F.L.; Neustrup, M.A.; Romeijn, S.; Galli, F.; Kuiper, J.; Jiskoot, W.; et al. Atomic force microscopy measurements of anionic liposomes reveal the effect of liposomal rigidity on antigen-specific regulatory T cell responses. J. Control. Release 2020, 318, 246–255. [Google Scholar] [CrossRef]
- Nagy, N.A.; de Haas, A.M.; Geijtenbeek, T.B.H.; van Ree, R.; Tas, S.W.; van Kooyk, Y.; de Jong, E.C. Therapeutic Liposomal Vaccines for Dendritic Cell Activation or Tolerance. Front. Immunol. 2021, 12, 674048. [Google Scholar] [CrossRef] [PubMed]
- Capurso, N.A.; Look, M.; Jeanbart, L.; Nowyhed, H.; Craft, J.; Abraham, C.; Fahmy, T.M. Development of a nanoparticulate formulation of retinoic acid that suppresses Th17 cells and upregulates regulatory T cells. Self/Nonself 2010, 1, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.E.; Garciafigueroa, Y.; Engman, C.; Liu, W.; Wang, Y.; Lakomy, R.J.; Meng, W.S.; Trucco, M.; Giannoukakis, N. Arrest in the Progression of Type 1 Diabetes at the Mid-Stage of Insulitic Autoimmunity Using an Autoanti-gen-Decorated All-trans Retinoic Acid and Transforming Growth Factor Beta-1 Single Microparticle Formulation. Front. Immunol. 2021, 12, 586220. [Google Scholar] [CrossRef] [PubMed]








| Formulation | Z-Average Diameter (nm) | ζ-Potential (mV) | PDI | Encapsulation hPG (%) | Encapsulation RA (%) |
|---|---|---|---|---|---|
| hPG | 186.8 ± 11.2 | −47.7 ± 2.1 | 0.10 ± 0.05 | 57.3 ± 3.3 | - |
| hPG/RA | 183.7 ± 4.9 | −45.9 ± 0.9 | 0.07 ± 0.01 | 43.9 ± 4.5 | 79.5 ± 29.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ter Braake, D.; Benne, N.; Lau, C.Y.J.; Mastrobattista, E.; Broere, F. Retinoic Acid-Containing Liposomes for the Induction of Antigen-Specific Regulatory T Cells as a Treatment for Autoimmune Diseases. Pharmaceutics 2021, 13, 1949. https://doi.org/10.3390/pharmaceutics13111949
ter Braake D, Benne N, Lau CYJ, Mastrobattista E, Broere F. Retinoic Acid-Containing Liposomes for the Induction of Antigen-Specific Regulatory T Cells as a Treatment for Autoimmune Diseases. Pharmaceutics. 2021; 13(11):1949. https://doi.org/10.3390/pharmaceutics13111949
Chicago/Turabian Styleter Braake, Daniëlle, Naomi Benne, Chun Yin Jerry Lau, Enrico Mastrobattista, and Femke Broere. 2021. "Retinoic Acid-Containing Liposomes for the Induction of Antigen-Specific Regulatory T Cells as a Treatment for Autoimmune Diseases" Pharmaceutics 13, no. 11: 1949. https://doi.org/10.3390/pharmaceutics13111949