Anti-Inflammatory Effect of Tacrolimus/Hydroxypropyl-β-Cyclodextrin Eye Drops in an Endotoxin-Induced Uveitis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation Elaboration Procedure
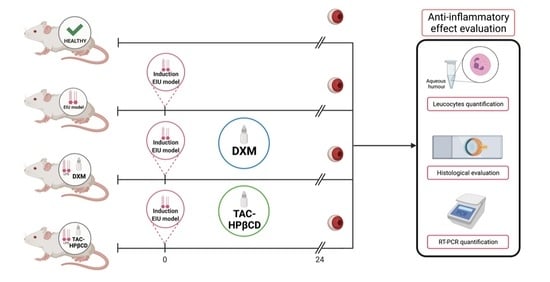
2.2. In Vivo Endotoxin-Induced Uveitis Model (EIU Model)
2.3. RNA Isolation and Real-Time PCR Analysis
2.4. Quantitative Analysis of Leucocytes in Aqueous Humor
2.5. Histological Evaluation
2.6. Statistical Analysis
3. Results
3.1. Evaluation of the Effect of Tacrolimus on IL-6, IL-8, MIP-1α, and TNF-α
3.2. Quantitative Analysis of Leucocytes in Aqueous Humor
3.3. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsirouki, T.; Dastiridou, A.; Dastiridou, O.; Brazitikou, I.; Kalogeropoulos, C.; Androudi, S. A Focus on the Epidemiology of Uveitis. Ocul. Immunol. Inflamm. 2018, 26, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Fonollosa, A.; Adán, A. Uveitis: A multidisciplinary approach. Arch. Soc. Esp. Oftalmol. 2011, 86, 393–394. [Google Scholar] [CrossRef]
- Acharya, N.R.; Tham, V.M.; Esterberg, E.; Borkar, D.S.; Parker, J.V.; Vinoya, A.C.; Uchida, A. Incidence and Prevalence of Uveitis: Results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013, 131, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.-K.; Chou, Y.-J.; Pu, C.-Y.; Chou, P. Epidemiology of Uveitis among the Chinese Population in Taiwan: A Population-Based Study. Ophthalmology 2012, 119, 2371–2376. [Google Scholar] [CrossRef]
- Hart, C.T.; Zhu, E.Y.; Crock, C.; Rogers, S.L.; Lim, L.L. Epidemiology of Uveitis in Urban Australia. Clin. Exp. Ophthalmol. 2019, 47, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Fanlo, P.; Heras, H.; Espinosa, G.; Adan, A. Complications and Visual Acuity of Patients with Uveitis: Epidemiological Study in a Reference Unit in Northern Spain. Arch. Soc. Esp. Oftalmol. 2019, 94, 419–425. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Pepple, K.L. Cytokines in Uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- De Smet, M.D.; Taylor, S.R.J.; Bodaghi, B.; Miserocchi, E.; Murray, P.I.; Pleyer, U.; Zierhut, M.; Barisani-Asenbauer, T.; LeHoang, P.; Lightman, S. Understanding Uveitis: The Impact of Research on Visual Outcomes. Prog. Retin. Eye Res. 2011, 30, 452–470. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.W.; Nicholson, L.B.; Sen, H.N.; Chan, C.-C.; Wei, L.; Nussenblatt, R.B.; Dick, A.D. Autoimmune and Autoinflammatory Mechanisms in Uveitis. Semin. Immunopathol. 2014, 36, 581–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.W. Ocular Immune Privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, S.; Das, D.; Hasanreisoglu, M.; Toy, B.C.; Akhter, M.; Anuradha, V.K.; Anthony, E.; Gurnani, B.; Kaur, K. Interleukins and Cytokine Biomarkers in Uveitis. Indian J. Ophthalmol. 2020, 68, 1750–1763. [Google Scholar] [CrossRef]
- Curnow, S.J.; Falciani, F.; Durrani, O.M.; Cheung, C.M.G.; Ross, E.J.; Wloka, K.; Rauz, S.; Wallace, G.R.; Salmon, M.; Murray, P.I. Multiplex Bead Immunoassay Analysis of Aqueous Humor Reveals Distinct Cytokine Profiles in Uveitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4251–4259. [Google Scholar] [CrossRef]
- Kirino, Y.; Bertsias, G.; Ishigatsubo, Y.; Mizuki, N.; Tugal-Tutkun, I.; Seyahi, E.; Ozyazgan, Y.; Sacli, F.S.; Erer, B.; Inoko, H.; et al. Genome-Wide Association Analysis Identifies New Susceptibility Loci for Behçet’s Disease and Epistasis between HLA-B*51 and ERAP1. Nat. Genet. 2013, 45, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Medawar, P.B. Immunity to Homologous Grafted Skin; the Fate of Skin Homografts Transplanted to the Brain, to Subcutaneous Tissue, and to the Anterior Chamber of the Eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar] [PubMed]
- Hou, S.; Du, L.; Lei, B.; Pang, C.P.; Zhang, M.; Zhuang, W.; Zhang, M.; Huang, L.; Gong, B.; Wang, M.; et al. Genome-Wide Association Analysis of Vogt-Koyanagi-Harada Syndrome Identifies Two New Susceptibility Loci at 1p31.2 and 10q21.3. Nat. Genet. 2014, 46, 1007–1011. [Google Scholar] [CrossRef]
- Dick, A.D.; Rosenbaum, J.T.; Al-Dhibi, H.A.; Belfort, R.; Brézin, A.P.; Chee, S.P.; Davis, J.L.; Ramanan, A.V.; Sonoda, K.-H.; Carreño, E.; et al. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis. Ophthalmology 2018, 125, 757–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahab, M.A.; Mir, T.A.; Zafar, S. Optimising Drug Therapy for Non-Infectious Uveitis. Int. Ophthalmol. 2019, 39, 1633–1650. [Google Scholar] [CrossRef] [PubMed]
- Valdes, L.M.; Sobrin, L. Uveitis Therapy: The Corticosteroid Options. Drugs 2020, 80, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Rosenbaum, J.T.; Foster, C.S.; Holland, G.N.; Jaffe, G.J.; Louie, J.S.; Nussenblatt, R.B.; Stiehm, E.R.; Tessler, H.; Gelder, R.N.V.; et al. Guidelines for the Use of Immunosuppressive Drugs in Patients with Ocular Inflammatory Disorders: Recommendations of an Expert Panel. Am. J. Ophthalmol. 2000, 130, 492–513. [Google Scholar] [CrossRef]
- Joseph, A.; Raj, D.; Shanmuganathan, V.; Powell, R.J.; Dua, H.S. Tacrolimus Immunosuppression in High-Risk Corneal Grafts. Br. J. Ophthalmol. 2007, 91, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caputo, R.; Marziali, E.; de Libero, C.; Di Grande, L.; Danti, G.; Virgili, G.; Villani, E.; Mori, F.; Bacci, G.M.; Lucenteforte, E.; et al. Long-Term Safety and Efficacy of Tacrolimus 0.1% in Severe Pediatric Vernal Keratoconjunctivitis. Cornea 2021, 40, 1395–1401. [Google Scholar] [CrossRef]
- Luaces-Rodríguez, A.; Touriño-Peralba, R.; Alonso-Rodríguez, I.; García-Otero, X.; González-Barcia, M.; Rodríguez-Ares, M.T.; Martínez-Pérez, L.; Aguiar, P.; Gómez-Lado, N.; Silva-Rodríguez, J.; et al. Preclinical Characterization and Clinical Evaluation of Tacrolimus Eye Drops. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 120, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Shoughy, S.S.; Jaroudi, M.O.; Tabbara, K.F. Efficacy and Safety of Low-Dose Topical Tacrolimus in Vernal Keratoconjunctivitis. Clin. Ophthalmol. Auckl. NZ 2016, 10, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astellas Pharma Inc A Randomized, Placebo-Controlled, Double-Masked Study of 0.1% Tacrolimus (FK506) Ophthalmic Suspension in Vernal Keratoconjunctivitis. Available online: clinicaltrials.gov (accessed on 17 August 2021).
- Moawad, P.; Shamma, R.; Hassanein, D.; Ragab, G.; El Zawahry, O. Evaluation of the Effect of Topical Tacrolimus 0.03% versus Cyclosporine 0.05% in the Treatment of Dry Eye Secondary to Sjogren Syndrome. Eur. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Wong, S.M.; Leung, A.T.S.; Leung, G.Y.S.; Cheng, L.L.; Lam, D.S.C. Successful Treatment of Surgically Induced Necrotizing Scleritis with Tacrolimus. Clin. Exp. Ophthalmol. 2005, 33, 98–99. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.W.; Seo, K.Y. Application for Tacrolimus Ointment in Treating Refractory Inflammatory Ocular Surface Diseases. Am. J. Ophthalmol. 2013, 155, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of Action of Tacrolimus (FK506): Molecular and Cellular Mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Murphy, C.C.; Greiner, K.; Plskova, J.; Duncan, L.; Frost, N.A.; Forrester, J.V.; Dick, A.D. Cyclosporine vs. Tacrolimus Therapy for Posterior and Intermediate Uveitis. Arch. Ophthalmol. Chic. 2005, 123, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horai, R.; Caspi, R.R. Cytokines in Autoimmune Uveitis. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2011, 31, 733–744. [Google Scholar] [CrossRef]
- Sakamoto, T. Cell biology of hyalocytes. Nippon Ganka Gakkai Zasshi 2003, 107, 866–882. [Google Scholar]
- Mesquida, M.; Molins, B.; Llorenç, V.; de la Maza, M.S.; Adán, A. Targeting Interleukin-6 in Autoimmune Uveitis. Autoimmun. Rev. 2017, 16, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Sloper, C.M.; Powell, R.J.; Dua, H.S. Tacrolimus (FK506) in the Treatment of Posterior Uveitis Refractory to Cyclosporine. Ophthalmology 1999, 106, 723–728. [Google Scholar] [CrossRef]
- Oh-i, K.; Keino, H.; Goto, H.; Yamakawa, N.; Murase, K.; Usui, Y.; Kezuka, T.; Sakai, J.-I.; Takeuchi, M.; Usui, M. Intravitreal Injection of Tacrolimus (FK506) Suppresses Ongoing Experimental Autoimmune Uveoretinitis in Rats. Br. J. Ophthalmol. 2007, 91, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Hokama, H.; Katagiri, Y.; Goto, H.; Usui, M. Effects of Intravitreal Injection of Tacrolimus (FK506) in Experimental Uveitis. Curr. Eye Res. 2005, 30, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Li, Q.; Wan, T.; Liu, C.; Pan, W.; Wu, Z.; Zhang, G.; Pan, J.; Qin, M.; Lin, Y.; et al. Hyaluronic Acid-Coated Niosomes Facilitate Tacrolimus Ocular Delivery: Mucoadhesion, Precorneal Retention, Aqueous Humor Pharmacokinetics, and Transcorneal Permeability. Colloids Surf. B Biointerfaces 2016, 141, 28–35. [Google Scholar] [CrossRef]
- Garg, V.; Nirmal, J.; Riadi, Y.; Kesharwani, P.; Kohli, K.; Jain, G.K. Amelioration of Endotoxin-Induced Uveitis in Rabbit by Topical Administration of Tacrolimus Proglycosome Nano-Vesicles. J. Pharm. Sci. 2021, 110, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Siegl, C.; König-Schuster, M.; Nakowitsch, S.; Koller, C.; Graf, P.; Unger-Manhart, N.; Schindlegger, Y.; Kirchoff, N.; Knecht, C.; Prieschl-Grassauer, E.; et al. Pharmacokinetics of Topically Applied Tacrolimus Dissolved in Marinosolv, a Novel Aqueous Eye Drop Formulation. Eur. J. Pharm. Biopharm. 2019, 134, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical Tacrolimus Nanocapsules Eye Drops for Therapeutic Effect Enhancement in Both Anterior and Posterior Ocular Inflammation Models. J. Control. Release 2021, 333, 283–297. [Google Scholar] [CrossRef]
- García-Otero, X.; Díaz-Tomé, V.; Varela-Fernández, R.; Martín-Pastor, M.; González-Barcia, M.; Blanco-Méndez, J.; Mondelo-García, C.; Bermudez, M.A.; Gonzalez, F.; Aguiar, P.; et al. Development and Characterization of a Tacrolimus/Hydroxypropyl-β-Cyclodextrin Eye Drop. Pharmaceutics 2021, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Cyclodextrins Used as Excipients. Available online: https://www.ema.europa.eu/en/cyclodextrins (accessed on 17 August 2021).
- The Association for Research in Vision and Ophthalmology-Statement for the Use of Animals in Ophthalmic and Vision Research. Available online: https://www.arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/ (accessed on 5 May 2021).
- National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals Guide for the Care and Use of Laboratory Animals. In The National Academies Collection: Reports Funded by National Institutes of Health, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Da Silva, P.S.; Girol, A.P.; Oliani, S.M. Mast Cells Modulate the Inflammatory Process in Endotoxin-Induced Uveitis. Mol. Vis. 2011, 17, 1310–1319. [Google Scholar]
- Girol, A.P.; Mimura, K.K.O.; Drewes, C.C.; Bolonheis, S.M.; Solito, E.; Farsky, S.H.P.; Gil, C.D.; Oliani, S.M. Anti-Inflammatory Mechanisms of the Annexin A1 Protein and Its Mimetic Peptide Ac2-26 in Models of Ocular Inflammation in Vivo and in Vitro. J. Immunol. 2013, 190, 5689–5701. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.; Perez-Fernandez, R. Anti-Inflammatory Effect of Conditioned Medium from Human Uterine Cervical Stem Cells in Uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef]
- Cury Martins, J.; Martins, C.; Aoki, V.; Gois, A.F.T.; Ishii, H.A.; da Silva, E.M.K. Topical Tacrolimus for Atopic Dermatitis. Cochrane Database Syst. Rev. 2015, CD009864. [Google Scholar] [CrossRef]
- Gamalero, L.; Simonini, G.; Ferrara, G.; Polizzi, S.; Giani, T.; Cimaz, R. Evidence-Based Treatment for Uveitis. Isr. Med. Assoc. J. IMAJ 2019, 21, 475–479. [Google Scholar]
- Hogan, A.C.; McAvoy, C.E.; Dick, A.D.; Lee, R.W.J. Long-Term Efficacy and Tolerance of Tacrolimus for the Treatment of Uveitis. Ophthalmology 2007, 114, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Yeom, D.; Kim, N.A.; Choi, D.H.; Park, J.; Wang, H.; Yoo, S.D.; Jeong, S.H. Bioequivalence of Tacrolimus Formulations with Different Dynamic Solubility and In-Vitro Dissolution Profiles. Arch. Pharm. Res. 2015, 38, 73–80. [Google Scholar] [CrossRef]
- Loftsson, T.; Muellertz, A.; Siepmann, J. For the Special IJP Issue “Poorly Soluble Drugs”. Int. J. Pharm. 2013, 453, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yu, J.M.; Ko, J.H. Analysis of Ethanol Effects on Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3852–3856. [Google Scholar] [CrossRef] [Green Version]
- Yadav, U.C.S.; Ramana, K.V. Endotoxin-Induced Uveitis in Rodents. Methods Mol. Biol. 2019, 1960, 161–168. [Google Scholar] [CrossRef]
- Fox, A.; Hammer, M.E.; Lill, P.; Burch, T.G.; Burrish, G. Experimental Uveitis. Elicited by Peptidoglycan-Polysaccharide Complexes, Lipopolysaccharide, and Muramyl Dipeptide. Arch. Ophthalmol. Chic. 1984, 102, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Vafadari, R.; Kraaijeveld, R.; Weimar, W.; Baan, C.C. Tacrolimus Inhibits NF-ΚB Activation in Peripheral Human T Cells. PLoS ONE 2013, 8, e60784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikita, N.; Chan, C.C.; Whitcup, S.M.; Nussenblatt, R.B.; Mochizuki, M. Effects of Topical FK506 on Endotoxin-Induced Uveitis (EIU) in the Lewis Rat. Curr. Eye Res. 1995, 14, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Mesquida, M.; Molins, B.; Llorenç, V.; Sainz de la Maza, M.; Hernandez, M.V.; Espinosa, G.; Adán, A. Proinflammatory Cytokines and C-Reactive Protein in Uveitis Associated with Behçet’s Disease. Mediat. Inflamm. 2014, 2014, e396204. [Google Scholar] [CrossRef] [PubMed]





| Primers | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-6 | CTTCAGGCCAAGTTCAGGAG | AGTGG ATCGTGGTCGTCTTC |
| IL-8 | TCCAGCCAGTTGCCTTCTTG | GGTCTGTTGTGGGTGGTATCC |
| MIP-1α | CGTCCTCATCCTGATCACCT | GATACATCCCCGTGAACACC |
| TNF-α | CCAGATGGTCACCCTCAGAT | CCTTGACCG CTGAAGAGAAC |
| 18S | GTAACCGCTTGAACCCCATT | CCATCCAATCGCTAGTAGCG |
| Tukey’s Multiple Comparison Test | IL-6 | IL-8 | MIP-1α | TNF-α |
|---|---|---|---|---|
| Healthy vs. EIU | YES ** | YES * | YES ** | YES ** |
| Healthy vs. TAC-HPβCD | NO | NO | NO | NO |
| Healthy vs. DXM | NO | NO | NO | YES ** |
| EIU vs. TAC-HPβCD | YES ** | NO | YES ** | YES ** |
| EIU vs. DXM | YES ** | NO | YES ** | YES * |
| TAC-HPβCD vs. DXM | NO | NO | NO | NO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Otero, X.; Mondelo-García, C.; González, F.; Perez-Fernandez, R.; Avila, L.; Antúnez-López, J.R.; González-Barcia, M.; Adan, A.; Aguiar, P.; Otero-Espinar, F.J.; et al. Anti-Inflammatory Effect of Tacrolimus/Hydroxypropyl-β-Cyclodextrin Eye Drops in an Endotoxin-Induced Uveitis Model. Pharmaceutics 2021, 13, 1737. https://doi.org/10.3390/pharmaceutics13101737
García-Otero X, Mondelo-García C, González F, Perez-Fernandez R, Avila L, Antúnez-López JR, González-Barcia M, Adan A, Aguiar P, Otero-Espinar FJ, et al. Anti-Inflammatory Effect of Tacrolimus/Hydroxypropyl-β-Cyclodextrin Eye Drops in an Endotoxin-Induced Uveitis Model. Pharmaceutics. 2021; 13(10):1737. https://doi.org/10.3390/pharmaceutics13101737
Chicago/Turabian StyleGarcía-Otero, Xurxo, Cristina Mondelo-García, Francisco González, Roman Perez-Fernandez, Leandro Avila, Jose Ramón Antúnez-López, Miguel González-Barcia, Alfredo Adan, Pablo Aguiar, Francisco J. Otero-Espinar, and et al. 2021. "Anti-Inflammatory Effect of Tacrolimus/Hydroxypropyl-β-Cyclodextrin Eye Drops in an Endotoxin-Induced Uveitis Model" Pharmaceutics 13, no. 10: 1737. https://doi.org/10.3390/pharmaceutics13101737