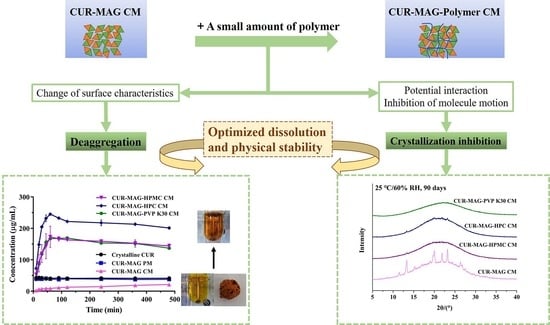
Deaggregation and Crystallization Inhibition by Small Amount of Polymer Addition for a Co-Amorphous Curcumin-Magnolol System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drug-Drug/Polymer Miscibility Using Solubility Parameters (δ)
2.3. Preparation of Co-Amorphous Systems
2.4. X-ray Powder Diffraction (XRPD)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Raman Spectroscopy
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Solid-State 13C Nuclear Magnetic Resonance Spectroscopy (ss 13C NMR)
2.9. Dissolution under Supersaturated Conditions
2.10. Contact Angle Measurements and Surface Free Energy Calculation
2.11. Nucleation Inhibition of CUR and MAG by Polymer
2.12. Broadband Dielectric Spectroscopy (BDS)
2.13. Physical Stability Evaluation
3. Results and Discussion
3.1. Drug-Drug/Polymer Miscibility Using Solubility Parameters (δ)
3.2. Preparation of Co-Amorphous Systems
3.3. Solid-State Characterization
3.3.1. XRPD
3.3.2. DSC
3.3.3. Raman Spectroscopy
3.3.4. FTIR
3.3.5. ss 13C NMR
3.4. Dissolution under Supersaturated Conditions
3.5. Contact Angle Measurements and Surface Free Energy Calculation
3.6. Nucleation Inhibitory Effect on CUR and MAG by Polymer
3.7. BDS
3.8. Physical Stability Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.; Wei, Y.; Lu, Y.; Wang, R.; Zhang, J.; Gao, Y.; Qian, S. Co-amorphous systems for the delivery of poorly water-soluble drugs: Recent advances and an update. Expert Opin. Drug Deliv. 2020, 17, 1411–1435. [Google Scholar] [CrossRef]
- Shi, Q.; Moinuddin, S.M.; Cai, T. Advances in coamorphous drug delivery systems. Acta Pharm. Sin. B 2019, 9, 19–35. [Google Scholar] [CrossRef]
- Qian, S.; Heng, W.L.; Wei, Y.F.; Zhang, J.J.; Gao, Y. Coamorphous lurasidone hydrochloride-saccharin with charge-assisted hydrogen bonding interaction shows improved physical stability and enhanced dissolution with pH-independent solubility behavior. Cryst. Growth Des. 2015, 15, 2920–2928. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, M.; Liu, T.; Gao, Z.; Ye, Q.; Tan, X.; Hou, Y.; Sun, J.; Wang, D.; He, Z. Co-amorphous solid dispersion systems of lacidipine-spironolactone with improved dissolution rate and enhanced physical stability. Asian J. Pharm. Sci. 2019, 14, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Lobmann, K.; Grohganz, H.; Rades, T. Co-former selection for co-amorphous drug-amino acid formulations. Int. J. Pharm. 2019, 557, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Song, S.; Ding, Z.; Fan, B.; Huang, W.; Xu, T. Improving the solubility, dissolution, and bioavailability of ibrutinib by preparing it in a coamorphous state with saccharin. J. Pharm. Sci. 2019, 108, 3020–3028. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lobmann, K.; Rades, T.; Grohganz, H. On the role of salt formation and structural similarity of co-formers in co-amorphous drug delivery systems. Int. J. Pharm. 2018, 535, 86–94. [Google Scholar] [CrossRef]
- Fan, N.; He, Z.; Ma, P.; Wang, X.; Li, C.; Sun, J.; Sun, Y.; Li, J. Impact of HPMC on inhibiting crystallization and improving permeability of curcumin amorphous solid dispersions. Carbohydr. Polym. 2018, 181, 543–550. [Google Scholar] [CrossRef]
- Fan, N.; Ma, P.; Wang, X.; Li, C.; Zhang, X.; Zhang, K.; Li, J.; He, Z. Storage stability and solubilization ability of HPMC in curcumin amorphous solid dispersions formulated by Eudragit E100. Carbohydr. Polym. 2018, 199, 492–498. [Google Scholar] [CrossRef]
- Liu, J.; Grohganz, H.; Rades, T. Influence of polymer addition on the amorphization, dissolution and physical stability of co-amorphous systems. Int. J. Pharm. 2020, 588, 119768. [Google Scholar] [CrossRef]
- Ruponen, M.; Visti, M.; Ojarinta, R.; Laitinen, R. Permeability of glibenclamide through a PAMPA membrane: The effect of co-amorphization. Eur. J. Pharm. Biopharm. 2018, 129, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Franca, M.T.; Marcos, T.M.; Pereira, R.N.; Stulzer, H.K. Could the small molecules such as amino acids improve aqueous solubility and stabilize amorphous systems containing Griseofulvin? Eur. J. Pharm. Sci. 2019, 143, 105178. [Google Scholar] [CrossRef]
- Bohr, A.; Nascimento, T.L.; Harmankaya, N.; Weisser, J.J.; Wang, Y.; Grohganz, H.; Rades, T.; Lobmann, K. Efflux inhibitor bicalutamide increases oral bioavailability of the poorly soluble efflux substrate docetaxel in co-amorphous anti-cancer combination therapy. Molecules 2019, 24, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapik-Kowalczuk, J.; Chmiel, K.; Jurkiewicz, K.; Correia, N.T.; Sawicki, W.; Paluch, M. Physical stability and viscoelastic properties of co-amorphous ezetimibe/simvastatin system. Pharmaceuticals 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, M.H.; DeVault, M.; Kuwata, K.T.; Suryanarayanan, R. Drug-excipient interactions: Effect on molecular mobility and physical stability of ketoconazole-organic acid coamorphous systems. Mol. Pharm. 2018, 15, 1052–1061. [Google Scholar] [CrossRef]
- Ueda, H.; Kadota, K.; Imono, M.; Ito, T.; Kunita, A.; Tozuka, Y. Co-amorphous formation induced by combination of tranilast and diphenhydramine hydrochloride. J. Pharm. Sci. 2017, 106, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, Y.; Ueda, H.; Wakabayashi, R.; Kamiya, N.; Goto, M. A novel binary supercooled liquid formulation for transdermal drug delivery. Biol. Pharm. Bull. 2019, 43, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.G.; Baldoni, H.A.; Davila, Y.A.; Brusau, E.V.; Ellena, J.A.; Narda, G.E. Rational design of a famotidine-ibuprofen coamorphous system: An experimental and theoretical study. J. Phys. Chem. B 2018, 122, 8772–8782. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Han, J.; Wang, R.; Zhao, X.; Qiao, H.; Chen, L.; Li, W.; Di, L.; Zhang, W.; Li, J. Surfactant-free solid dispersion of BCS class IV drug in an amorphous chitosan oligosaccharide matrix for concomitant dissolution in vitro—Permeability increase. Eur. J. Pharm. Sci. 2019, 130, 147–155. [Google Scholar] [CrossRef]
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017, 85, 102–112. [Google Scholar] [CrossRef]
- Chen, C.H.; Hsu, F.T.; Chen, W.L.; Chen, J.H. Induction of apoptosis, inhibition of MCL-1, and VEGF-A expression are associated with the anti-cancer efficacy of magnolol combined with regorafenib in hepatocellular carcinoma. Cancers 2021, 13, 2066. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, toxicity, bioavailability, and formulation of magnolol: An update. Front. Pharmacol. 2021, 12, 632767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yu, Z.; Feng, L.; Deng, L.; Fang, Z.; Liu, Z.; Li, Y.; Wu, X.; Qin, L.; Guo, R.; et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021, 268, 118237. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, B.; Ali, M.N.; Riaz, Z. Synthesis and appraisal of natural drug-polymer-based matrices relevant to the application of drug-eluting coronary stent coatings. Cardiol. Res. Pract. 2020, 2020, 4073091. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Araiza, C.; Alvarez-Mejia, A.L.; Sanchez-Torres, S.; Farfan-Garcia, E.; Mondragon-Lozano, R.; Pinto-Almazan, R.; Salgado-Ceballos, H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013, 47, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Dandapat, J.; Dash, U.C.; Kanhar, S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018, 215, 42–73. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Xiao, L.; Zhu, L.; Li, W.; Li, C.; Cao, Y.; Ge, G.; Sun, X. New insights into SN-38 glucuronidation: Evidence for the important role of UDP glucuronosyltransferase 1A9. Basic Clin. Pharmacol. Toxicol. 2018, 122, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Ge, G.; Zhang, H.; Liu, H.; He, G.; Liang, S.; Zhang, Y.; Fang, Z.; Dong, P.; Finel, M.; et al. Characterization of hepatic and intestinal glucuronidation of magnolol: Application of the relative activity factor approach to decipher the contributions of multiple UDP-glucuronosyltransferase isoforms. Drug Metab. Dispos. 2012, 40, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Metre, S.; Mukesh, S.; Samal, S.K.; Chand, M.; Sangamwar, A.T. Enhanced biopharmaceutical performance of rivaroxaban through polymeric amorphous solid dispersion. Mol. Pharm. 2018, 15, 652–668. [Google Scholar] [CrossRef]
- DeBoyace, K.; Wildfong, P.L.D. The application of modeling and prediction to the formation and stability of amorphous solid dispersions. J. Pharm. Sci. 2018, 107, 57–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korhonen, O.; Pajula, K.; Laitinen, R. Rational excipient selection for co-amorphous formulations. Expert Opin. Drug Deliv. 2017, 14, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Co amorphous systems: A product development perspective. Int. J. Pharm. 2016, 515, 403–415. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, H.; Babu, S.; Garad, S. Co-amorphous formation of high-dose zwitterionic compounds with amino acids to improve solubility and enable parenteral delivery. Mol. Pharm. 2018, 15, 97–107. [Google Scholar] [CrossRef]
- Pacult, J.; Rams-Baron, M.; Chmiel, K.; Jurkiewicz, K.; Antosik, A.; Szafraniec, J.; Kurek, M.; Jachowicz, R.; Paluch, M. How can we improve the physical stability of co-amorphous system containing flutamide and bicalutamide? The case of ternary amorphous solid dispersions. Eur. J. Pharm. Sci. 2019, 136, 104947. [Google Scholar] [CrossRef] [PubMed]
- Zdziennicka, A.; Szymczyk, K.; Janczuk, B. Correlation between surface free energy of quartz and its wettability by aqueous solutions of nonionic, anionic and cationic surfactants. J. Colloid Interface Sci. 2009, 340, 243–248. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B. Surface and thermal properties of collagen/hyaluronic acid blends containing chitosan. Int. J. Biol. Macromol. 2016, 92, 371–376. [Google Scholar] [CrossRef]
- Krawczyk, J. Surface free energy of the human skin and its critical surface tension of wetting in the skin/surfactant aqueous solution/air system. Skin Res. Technol. 2015, 21, 214–223. [Google Scholar] [CrossRef]
- Rojewska, M.; Bartkowiak, A.; Strzemiecka, B.; Jamrozik, A.; Voelkel, A.; Prochaska, K. Surface properties and surface free energy of cellulosic etc mucoadhesive polymers. Carbohydr. Polym. 2017, 171, 152–162. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Yakup Arica, M. Surface energy components of a dye-ligand immobilized pHEMA membranes: Effects of their molecular attracting forces for non-covalent interactions with IgG and HSA in aqueous media. Int. J. Biol. Macromol. 2005, 37, 249–256. [Google Scholar] [CrossRef]
- Edueng, K.; Mahlin, D.; Larsson, P.; Bergstrom, C.A.S. Mechanism-based selection of stabilization strategy for amorphous formulations: Insights into crystallization pathways. J. Control. Release 2017, 256, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Nagy, S.; Pal, S.; Szechenyi, A. Reliability of the Hansen solubility parameters as co-crystal formation prediction tool. Int. J. Pharm. 2019, 558, 319–327. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013–5015. [Google Scholar] [CrossRef] [PubMed]
- Matlinska, M.A.; Wasylishen, R.E.; Bernard, G.M.; Terskikh, V.V.; Brinkmann, A.; Michaelis, V.K. Capturing elusive polymorphs of curcumin: A structural characterization and computational study. Cryst. Growth Des. 2018, 18, 5556–5563. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G. Curcumin, a biological wonder molecule: A crystal engineering point of view. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Wang, R.; Han, J.; Jiang, A.; Huang, R.; Fu, T.; Wang, L.; Zheng, Q.; Li, W.; Li, J. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int. J. Pharm. 2019, 561, 9–18. [Google Scholar] [CrossRef]
- Luebbert, C.; Stoyanov, E.; Sadowski, G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. 2021, 3, 100070. [Google Scholar]
- Safna Hussan, K.P.; Thayyil, M.S.; Deshpande, S.K.; Jinitha, T.V.; Manoj, K.; Ngai, K.L. Molecular dynamics, physical and thermal stability of neat amorphous amlodipine besylate and in binary mixture. Eur. J. Pharm. Sci. 2018, 119, 268–278. [Google Scholar] [CrossRef]
- Fan, N.; Lu, T.; Li, J. Surface tracking of curcumin amorphous solid dispersions formulated by binary polymers. J. Pharm. Sci. 2020, 109, 1068–1078. [Google Scholar] [CrossRef]
- Minecka, A.; Kaminska, E.; Heczko, D.; Jurkiewicz, K.; Wolnica, K.; Dulski, M.; Hachula, B.; Pisarski, W.; Tarnacka, M.; Talik, A.; et al. Studying structural and local dynamics in model H-bonded active ingredient—Curcumin in the supercooled and glassy states at various thermodynamic conditions. Eur. J. Pharm. Sci. 2019, 135, 38–50. [Google Scholar] [CrossRef]
- Yu, D.Q.; Han, X.J.; Shan, T.Y.; Xu, R.; Hu, J.; Cheng, W.X.; Zha, L.P.; Peng, H.S. Microscopic characteristic and chemical composition analysis of three medicinal plants and surface frosts. Molecules 2019, 24, 4548. [Google Scholar] [CrossRef] [Green Version]
- Kozbial, A.; Trouba, C.; Liu, H.T.; Li, L. Characterization of the intrinsic water wettability of graphite using contact angle measurements: Effect of defects on static and dynamic contact angles. Langmuir 2017, 33, 959–967. [Google Scholar] [CrossRef]
- Zhu, C.; Gao, Y.; Li, H.; Meng, S.; Li, L.; Francisco, J.S.; Zeng, X.C. Characterizing hydrophobicity of amino acid side chains in a protein environment via measuring contact angle of a water nanodroplet on planar peptide network. Proc. Natl. Acad. Sci. USA 2016, 113, 12946–12951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, M.A.; Zia, K.M.; Ilyas, H.N.; Yaqub, N.; Bhatti, I.A.; Rehan, M.; Shoaib, M.; Bahadur, A. Influence of chitosan/1,4-butanediol blends on the thermal and surface behavior of polycaprolactone diol-based polyurethanes. Int. J. Biol. Macromol. 2019, 141, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Yarce, C.J.; Pineda, D.; Correa, C.E.; Salamanca, C.H. Relationship between surface properties and in vitro drug release from a compressed matrix containing an amphiphilic polymer material. Pharmaceuticals 2016, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Tiraferri, A.; Li, T.; Xie, W.; Chang, H.; Bai, Y.; Liu, B. Superwettable PVDF/PVDF-g-PEGMA ultrafiltration membranes. ACS Omega 2020, 5, 23450–23459. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Stawski, T.M.; Morgan, D.J.; Peacock, C.L.; Benning, L.G. The effects of inorganic additives on the nucleation and growth kinetics of calcium sulfate dihydrate crystals. Cryst. Growth Des. 2017, 17, 582–589. [Google Scholar] [CrossRef]
- Pang, Z.; Weng, X.; Wei, Y.; Gao, Y.; Zhang, J.; Qian, S. Modification of hygroscopicity and tabletability of l-carnitine by a cocrystallization technique. CrystEngComm 2021, 23, 2138–2149. [Google Scholar] [CrossRef]
- Jackson, M.J.; Kestur, U.S.; Hussain, M.A.; Taylor, L.S. Characterization of supersaturated danazol solutions—Impact of polymers on solution properties and phase transitions. Pharm. Res. 2016, 33, 1276–1288. [Google Scholar] [CrossRef]
- Sarode, A.L.; Wang, P.; Obara, S.; Worthen, D.R. Supersaturation, nucleation, and crystal growth during single- and biphasic dissolution of amorphous solid dispersions: Polymer effects and implications for oral bioavailability enhancement of poorly water soluble drugs. Eur. J. Pharm. Biopharm. 2014, 86, 351–360. [Google Scholar] [CrossRef]
- Syromotina, D.S.; Surmenev, R.A.; Surmeneva, M.A.; Boyandin, A.N.; Nikolaeva, E.D.; Prymak, O.; Epple, M.; Ulbricht, M.; Oehr, C.; Volova, T.G. Surface wettability and energy effects on the biological performance of poly-3-hydroxybutyrate films treated with RF plasma. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 450–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminska, E.; Madejczyk, O.; Tarnacka, M.; Jurkiewicz, K.; Kaminski, K.; Paluch, M. Studying of crystal growth and overall crystallization of naproxen from binary mixtures. Eur. J. Pharm. Biopharm. 2017, 113, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Minecka, A.; Kaminska, E.; Tarnacka, M.; Jurkiewicz, K.; Talik, A.; Wolnica, K.; Dulski, M.; Kasprzycka, A.; Spychalska, P.; Garbacz, G.; et al. Does the molecular mobility and flexibility of the saccharide ring affect the glass-forming ability of naproxen in binary mixtures? Eur. J. Pharm. Sci. 2020, 141, 105091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Suo, Z.; Peng, X.; Gan, N.; Zhao, L.; Tang, P.; Wei, X.; Li, H. Microcrystalline cellulose as an effective crystal growth inhibitor for the ternary Ibrutinib formulation. Carbohydr. Polym. 2020, 229, 115476. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S. Tailoring supersaturation from amorphous solid dispersions. J. Control. Release 2018, 279, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Wu, H.; Cai, T. Effect of polymeric excipients on nucleation and crystal growth kinetics of amorphous fluconazole. Biomater. Sci. 2021, 9, 4308–4316. [Google Scholar] [CrossRef] [PubMed]














| Sample | Experimental Tg, °C | Calculated Tg, °C | ΔTg, °C |
|---|---|---|---|
| Amorphous CUR | 74.6 | ||
| Amorphous MAG | −16.4 | ||
| CUR-MAG CM | 21.1 | 29.5 | −8.4 |
| CUR-MAG-HPMC CM | 26.1 | 22.0 | 4.1 |
| CUR-MAG-HPC CM | 23.5 | 21.9 | 1.6 |
| CUR-MAG-PVP K30 CM | 37.4 | 22.0 | 15.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Li, L.; Su, M.; Heng, W.; Wei, Y.; Gao, Y.; Qian, S. Deaggregation and Crystallization Inhibition by Small Amount of Polymer Addition for a Co-Amorphous Curcumin-Magnolol System. Pharmaceutics 2021, 13, 1725. https://doi.org/10.3390/pharmaceutics13101725
Han J, Li L, Su M, Heng W, Wei Y, Gao Y, Qian S. Deaggregation and Crystallization Inhibition by Small Amount of Polymer Addition for a Co-Amorphous Curcumin-Magnolol System. Pharmaceutics. 2021; 13(10):1725. https://doi.org/10.3390/pharmaceutics13101725
Chicago/Turabian StyleHan, Jiawei, Luyuan Li, Meiling Su, Weili Heng, Yuanfeng Wei, Yuan Gao, and Shuai Qian. 2021. "Deaggregation and Crystallization Inhibition by Small Amount of Polymer Addition for a Co-Amorphous Curcumin-Magnolol System" Pharmaceutics 13, no. 10: 1725. https://doi.org/10.3390/pharmaceutics13101725