Minocycline-Derived Silver Nanoparticles for Assessment of Their Antidiabetic Potential against Alloxan-Induced Diabetic Mice
Abstract
:1. Introduction
2. Experiment
2.1. Ethical Statement
2.2. Materials
2.3. Synthesis of Minocycline-Derived Silver Nanoparticles
2.4. Characterization of the Minocycline-Modified Silver Nanoparticles
2.5. Antioxidant Study—DPPH Assay
2.6. Experimental Animals
2.7. Induction of Diabetes
2.8. Experimental Design
2.9. Collection of Sample
2.10. Biochemical Assay
2.11. Histopathological Studies
2.12. Statistical Analysis
3. Results and Discussion
3.1. Strategy of Assay
3.2. Synthesis and Stability of Minocycline-Modified Silver Nanoparticles
3.3. Characterization of Minocycline-Modified Silver Nanoparticles
3.4. DPPH Radical Scavenging Assay
3.5. Antihyperglycemic Activity of Minocycline-Modified Silver Nanoparticles in Alloxan-Induced Diabetic Mice
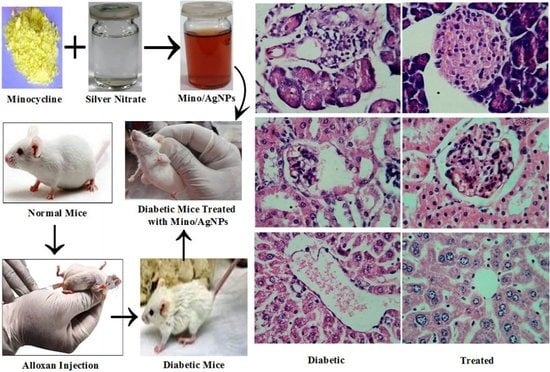
3.6. Histology Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Cai, H.; Liu, Z.; Yao, P. Effective Enhancement of Hypoglycemic Effect of Insulin by Liver-Targeted Nanoparticles Containing Cholic Acid-Modified Chitosan Derivative. Mol. Pharm. 2016, 13, 2433–2442. [Google Scholar] [CrossRef]
- Arvanag, F.M.; Bayrami, A.; Habibi-Yangjeh, A.; Pouran, S.R. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L. seed extract. Mater. Sci. Eng. C 2018, 97, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.; Attia, M.F.; El Bana, M.; El-Daly, S.; Mohamed, N.; El-Khayat, Z.; El-Naggar, M.E. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 2019, 140, 1305–1314. [Google Scholar] [CrossRef]
- Dhas, S.; Kumar, V.G.; Karthick, V.; Vasanth, K.; Kasivelu, G.; Govindaraju, K. Effect of biosynthesized gold nanoparticles by Sargassum swartzii in alloxan induced diabetic rats. Enzym. Microb. Technol. 2016, 95, 100–106. [Google Scholar] [CrossRef]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R.S. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2014, 14, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, E.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.J. Type 2 Diabetes—A Matter of—Cell Life and Death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Taylor, J.R. New Developments in Diabetes Management. Drug Top. 2010, 154, 32–39. [Google Scholar]
- Kazmi, S.A.R.; Qureshi, M.Z.; Masson, J.-F. Drug-Based Gold Nanoparticles Overgrowth for Enhanced SPR Biosensing of Doxycycline. Biosensors 2020, 10, 184. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Qin, J.; Nie, G.; Lei, B.; Xiao, Y.; Zheng, M.; Rong, J. Fabrication of Reduced Graphene Oxide and Sliver Nanoparticle Hybrids for Raman Detection of Absorbed Folic Acid: A Potential Cancer Diagnostic Probe. ACS Appl. Mater. Interfaces 2013, 5, 4760–4768. [Google Scholar] [CrossRef]
- Zhou, B.; Xiong, Z.; Wang, P.; Peng, C.; Shen, M.; Shi, X. Acetylated Polyethylenimine-Entrapped Gold Nanoparticles Enable Negative Computed Tomography Imaging of Orthotopic Hepatic Carcinoma. Langmuir 2016, 34, 8701–8707. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Javed, S.; Qureshi, M.Z.; Khan, M.S.; Khan, M.U. Synthesis and study of catalytic application of l-methionine protected gold nanoparticles. Appl. Nanosci. 2017, 7, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Kazmi, S.A.R.; Qureshi, M.Z.; Ali, S.; Masson, J.-F. In Vitro Drug Release and Biocatalysis from pH-Responsive Gold Nanoparticles Synthesized Using Doxycycline. Langmuir 2019, 35, 16266–16274. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Kouame, K.; Peter, A.I.; Akang, E.N.; Moodley, R.; Naidu, E.C.; Azu, O.O. Histological and biochemical effects of Cinnamomum cassia nanoparticles in kidneys of diabetic Sprague-Dawley rats. Bosn. J. Basic Med. Sci. 2019, 19, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Park, E.-J.; Chun, I.K.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C. Bioavailability and Toxicokinetics of citrate-coated silver nanoparticles in rats. Arch. Pharmacal Res. 2011, 34, 153–158. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J. Trace Elements Med. Biol. 2017, 40, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Lamana, J.; Laborda, F.; Bolea, E.; Álvaro, I.A.; Castillo, J.R.; Bianga, J.; He, M.; Bierla, K.; Mounicou, S.; Ouerdane, L.; et al. An insight into silver nanoparticles bioavailability in rats. Metallomics 2014, 6, 2242–2249. [Google Scholar] [CrossRef] [Green Version]
- Ahn, E.-Y.; Jin, H.; Park, Y. Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C 2019, 101, 204–216. [Google Scholar] [CrossRef]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver Nanoparticles Mediated by Costus afer Leaf Extract: Synthesis, Antibacterial, Antioxidant and Electrochemical Properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küp, F.; Çoşkunçay, S.; Duman, F. Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater. Sci. Eng. C 2019, 107, 110207. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2017, 46, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.; Andersen, M.; Corkey, B.; et al. Reactive Oxygen Species as a Signal in Glucose-Stimulated Insulin Secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Kaneto, H.; Matsuoka, T.-A.; Katakami, N.; Kawamori, D.; Miyatsuka, T.; Yoshiuchi, K.; Yasuda, T.; Sakamoto, K.; Yamasaki, Y.; Matsuhisa, M. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr. Mol. Med. 2007, 7, 674–686. [Google Scholar] [CrossRef]
- Campoy, A.H.G.; Gutiérrez, R.M.P.; Manriquez-Alvirde, G.; Ramirez, A.M. Protection of silver nanoparticles using Eysenhardtia polystachya in peroxide-induced pancreatic β-Cell damage and their antidiabetic properties in zebrafish. Int. J. Nanomed. 2018, 13, 2601–2612. [Google Scholar] [CrossRef] [Green Version]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Pourgholami, M.H.; Mekkawy, A.; Badar, S.; Morris, D.L. Minocycline inhibits growth of epithelial ovarian cancer. Gynecol. Oncol. 2012, 125, 433–440. [Google Scholar] [CrossRef]
- Soory, M. A Role for Non-Antimicrobial Actions of Tetracyclines in Combating Oxidative Stress in Periodontal and Metabolic Diseases: A Literature Review. Open Dent. J. 2008, 2, 5–12. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Suzuki, S.; Fujisawa, S. Radical-scavenging and Pro-/anti-inflammatory Activity of Tetracycline and Related Phenolic Compounds with or Without Visible Light Irradiation. In Vivo 2019, 34, 81–94. [Google Scholar] [CrossRef]
- Lee, G.J.; Lim, J.J.; Hyun, S. Minocycline treatment increases resistance to oxidative stress and extends lifespan in Drosophila via FOXO. Oncotarget 2017, 8, 87878–87890. [Google Scholar] [CrossRef] [Green Version]
- Kraus, R.L.; Pasieczny, R.; Lariosa-Willingham, K.; Turner, M.S.; Jiang, A.; Trauger, J.W. Antioxidant properties of minocycline: Neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J. Neurochem. 2005, 94, 819–827. [Google Scholar] [CrossRef]
- Ali, S.; Bashir, S.; Mumtaz, S.; Shakir, H.A.; Ara, C.; Ahmad, F.; Tahir, H.M.; Faheem, M.; Irfan, M.; Masih, A.; et al. Evaluation of Cadmium Chloride-Induced Toxicity in Chicks Via Hematological, Biochemical Parameters, and Cadmium Level in Tissues. Biol. Trace Elem. Res. 2020, 199, 3457–3469. [Google Scholar] [CrossRef]
- Ara, C.; Asmatullah; Butt, N.; Ali, S.; Batool, F.; Shakir, H.A.; Arshad, A. Abnormal steroidogenesis, oxidative stress, and reprotoxicity following prepubertal exposure to butylparaben in mice and protective effect of Curcuma longa. Environ. Sci. Pollut. Res. 2020, 28, 6111–6121. [Google Scholar] [CrossRef]
- Mughal, T.A.; Ali, S.; Hassan, A.; Saleem, M.Z.; Mumtaz, S.; Mumtaz, S. Carbon Tetrachloride-Induced Hepatocellular Damage in Balb C Mice and Pharmacological Intervention by Extract of Daucus Carota. RADS J. Pharm. Pharm. Sci. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Naeem, S.; Ashraf, M.; Babar, M.E.; Zahoor, S.; Ali, S. The effects of some heavy metals on some fish species. Environ. Sci. Pollut. Res. 2021, 28, 25566–25578. [Google Scholar] [CrossRef]
- Ali, S.; Ejaz, M.; Dar, K.K.; Nasreen, S.; Ashraf, N.; Gillani, S.F.; Shafi, N.; Safeer, S.; Khan, M.A.; Andleeb, S.; et al. Evaluation of chemopreventive and chemotherapeutic effect of Artemisia vulgaris extract against diethylnitrosamine induced hepatocellular carcinogenesis in Balb C mice. Braz. J. Biol. 2020, 80, 484–496. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Ali, S.; Mumtaz, S.; Shakir, H.A.; Ahmad, F.; Tahir, H.M.; Ulhaq, M.; Khan, M.A.; Zahid, M.T. Dose and duration-dependent toxicological evaluation of lead acetate in chicks. Environ. Sci. Pollut. Res. 2020, 27, 15149–15164. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, S.; Khan, R.; Mumtaz, S.; Ashraf, N.; Andleeb, S.; Shakir, H.A.; Tahir, H.M.; Khan, M.K.A.; Ulhaq, M. Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ. Sci. Pollut. Res. 2018, 26, 3909–3920. [Google Scholar] [CrossRef] [PubMed]
- Dar, K.K.; Ali, S.; Ejaz, M.; Nasreen, S.; Ashraf, N.; Gillani, S.F.; Shafi, N.; Safeer, S.; Khan, M.A.; Andleeb, S.; et al. In vivo induction of hepatocellular carcinoma by diethylnitrosoamine and pharmacological intervention in Balb C mice using Bergenia ciliata extracts. Braz. J. Biol. 2019, 79, 629–638. [Google Scholar] [CrossRef]
- Mumtaz, S.; Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Mughal, T.A.; Younas, M. Evaluation of antibacterial activity of vitamin c against human bacterial pathogens. ResearchGate 2021, 83, 1–8. [Google Scholar]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2007, 51, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2018, 158, 8–14. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydari, R.; Rashidipour, M. Green Synthesis of Silver Nanoparticles Using Extract of Oak Fruit Hull (Jaft): Synthesis and In Vitro Cytotoxic Effect on MCF-7 Cells. Int. J. Breast Cancer 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- El-Gharbawy, R.M.; Emara, A.; Abu-Risha, S.E.-S. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed. Pharmacother. 2016, 84, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2020, 16, 62–76. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamaony, L.; Al-Khazraji, S.M.; Twaij, H.A. Hypoglycaemic effect of Artemisia herba alba. II. Effect of a valuable extract on some blood parameters in diabetic animals. J. Ethnopharmacol. 1994, 43, 167–171. [Google Scholar] [CrossRef]
- Ananthan, R.; Latha, M.; Ramkumar, K.M.; Pari, L.; Baskar, C.; Bai, V.N. Effect of Gymnema montanum leaves on serum and tissue lipids in alloxan diabetic rats. Exp. Diabesity Res. 2003, 4, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.; Kang, H.; Choi, H.; Choi, W.; Jun, H.-S. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell 2019, 18, e12895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.-L.; Shi, Y.-H.; Hao, G.; Li, W.; Le, G.-W. Increasing Oxidative Stress with Progressive Hyperlipidemia in Human: Relation between Malondialdehyde and Atherogenic Index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Daisy, P.; Eliza, J.; Ignacimuthu, S. Influence of Costus speciosus (Koen.) Sm. Rhizome Extracts on Biochemical Parameters in Streptozotocin Induced Diabetic Rats. J. Health Sci. 2008, 54, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Kaneto, H.; Kajimoto, Y.; Miyagawa, J.; Matsuoka, T.; Fujitani, Y.; Umayahara, Y.; Hanafusa, T.; Matsuzawa, Y.; Yamasaki, Y.; Hori, M. Beneficial effects of antioxidants in diabetes: Possible protection of pancreatic beta-cells against glucose toxicity. Diabetes 1999, 48, 2398–2406. [Google Scholar] [CrossRef]













| Treatments | Groups | Weight (g) at Various Time Points during Diabetes Treatment | ||||
|---|---|---|---|---|---|---|
| at 0 Day of Treatment | at 7th Day of Treatment | at 14th Day of Treatment | at 21st Day of Treatment | at 28th Day of Treatment | ||
| Normal Control | Group-I | 34.2 ± 0.45 | 34.6 ± 0.61 | 34.8 ± 0.37 | 35.1 ± 0.25 | 35.3 ± 0.49 |
| Diabetic | Group-II | 32.8 ± 0.74 | 31.4 ± 0.71 | 29.9 ± 0.58 # | 28.2 ± 0.40 ## | 26.0 ± 0.35 ### |
| Glibenclamide | Group-III | 33.0 ± 0.69 | 31.7 ± 0.45 | 32.1 ± 0.29 | 32.6 ± 0.18 $ | 32.9 ± 0.25 $$ |
| Mino/AgNPs | Group-IV | 33.4 ± 0.59 | 32.3 ± 0.65 | 32.9 ± 0.43 | 33.4 ± 0.27 ** | 33.8 ± 0.17 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazmi, S.A.R.; Qureshi, M.Z.; Sadia; Alhewairini, S.S.; Ali, S.; Khurshid, S.; Saeed, M.; Mumtaz, S.; Mughal, T.A. Minocycline-Derived Silver Nanoparticles for Assessment of Their Antidiabetic Potential against Alloxan-Induced Diabetic Mice. Pharmaceutics 2021, 13, 1678. https://doi.org/10.3390/pharmaceutics13101678
Kazmi SAR, Qureshi MZ, Sadia, Alhewairini SS, Ali S, Khurshid S, Saeed M, Mumtaz S, Mughal TA. Minocycline-Derived Silver Nanoparticles for Assessment of Their Antidiabetic Potential against Alloxan-Induced Diabetic Mice. Pharmaceutics. 2021; 13(10):1678. https://doi.org/10.3390/pharmaceutics13101678
Chicago/Turabian StyleKazmi, Syed Akif Raza, Muhammad Zahid Qureshi, Sadia, Saleh S. Alhewairini, Shaukat Ali, Shazia Khurshid, Muhammad Saeed, Shumaila Mumtaz, and Tafail Akbar Mughal. 2021. "Minocycline-Derived Silver Nanoparticles for Assessment of Their Antidiabetic Potential against Alloxan-Induced Diabetic Mice" Pharmaceutics 13, no. 10: 1678. https://doi.org/10.3390/pharmaceutics13101678