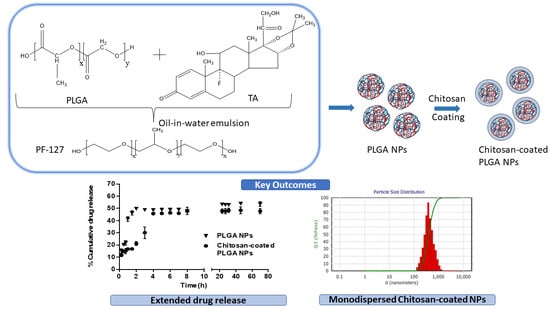
Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Optimization of Chitosan-Coated PLGA Nanoparticles
2.2.2. Particle Size, Polydispersity Index and Zeta Potential
2.2.3. Encapsulation Efficiency
2.2.4. Freeze-Drying of Nanoparticles
2.2.5. Thermal Analysis
2.2.6. Fourier-Transform Infrared Spectroscopy Analysis
2.2.7. In Vitro Drug Release
2.2.8. Statistical Analysis
3. Results
3.1. Screening of Chitosan-Coated PLGA Nanoparticles
3.2. Optimization of Nanoparticles Using Statistical Experimental Design
3.3. Response Optimization Using Response Surface Design
3.4. Thermal Analysis
3.5. Fourier Transform Infra-Red Spectroscopy (FT-IR) Analysis
3.6. In Vitro Drug Release Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Morley, E. Blindness and Vision. In Iris Murdoch and Elias Canetti: Intellectual Allies; Routledge: Oxfordshire, UK, 2018; Available online: https://www.who.int/en/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 15 June 2021).
- Eurostats. Statistical Office of the European Communities, Population Structure and Ageing—Statistics Explained: Luxembourg; Eurostats: Luxembourg, 2019; pp. 1–10. [Google Scholar]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senra, H.; Balaskas, K.; Mahmoodi, N.; Aslam, T. Experience of Anti-VEGF Treatment and Clinical Levels of Depression and Anxiety in Patients with Wet Age-Related Macular Degeneration. Am. J. Ophthalmol. 2017, 177, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Diabetic macular edema, retinopathy and age-related macular degeneration as inflammatory conditions. Arch. Med Sci. 2016, 12, 1142–1157. [Google Scholar] [CrossRef] [Green Version]
- Vandewalle, J.; Luypaert, A.; de Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Otsuka, T.; Naito, A.; Shimazawa, M.; Hara, H. Triamcinolone Acetonide Suppresses Inflammation and Facilitates Vascular Barrier Function in Human Retinal Microvascular Endothelial Cells. Curr. Neurovascular Res. 2017, 14, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Hirani, A.; Grover, A.; Lee, Y.W.; Pathak, Y.; Sutariya, V. Triamcinolone acetonide nanoparticles incorporated in thermoreversible gels for age-related macular degeneration. Pharm. Dev. Technol. 2014, 21, 61–67. [Google Scholar] [CrossRef]
- Sabzevari, A.; Adibkia, K.; Hashemi, H.; Hedayatfar, A.; Mohsenzadeh, N.; Atyabi, F.; Ghahremani, M.H.; Dinarvand, R. Polymeric triamcinolone acetonide nanoparticles as a new alternative in the treatment of uveitis: In vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2013, 84, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Birrenbach, G.; Speiser, P. Polymerized Micelles and Their Use as Adjuvants in Immunology. J. Pharm. Sci. 1976, 65, 1763–1766. [Google Scholar] [CrossRef]
- Yadav, M.; Schiavone, N.; Guzman-Aranguez, A.; Giansanti, F.; Papucci, L.; Perez De Lara, M.J.; Singh, M.; Kaur, I.P. Atorvastatin-loaded solid lipid nanoparticles as eye drops: Proposed treatment option for age-related macular degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 919–944. [Google Scholar] [CrossRef]
- Chittasupho, C.; Kengtrong, K.; Chalermnithiwong, S.; Sarisuta, N. Anti-angiogenesis by dual action of R5K peptide conjugated itraconazole nanoparticles. AAPS PharmSciTech 2020, 21, 74. [Google Scholar] [CrossRef]
- Shi, Z.; Li, S.K.; Charoenputtakun, P.; Liu, C.-Y.; Jasinski, D.; Guo, P. RNA nanoparticle distribution and clearance in the eye after subconjunctival injection with and without thermosensitive hydrogels. J. Control. Release 2018, 270, 14–22. [Google Scholar] [CrossRef]
- Li, C.; Chen, R.; Xu, M.; Qiao, J.; Yan, L.; Guo, X.D. Hyaluronic acid modified MPEG-b-PAE block copolymer aqueous micelles for efficient ophthalmic drug delivery of hydrophobic genistein. Drug Deliv. 2018, 25, 1258–1265. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Yu, S.; Pan, H.; Li, J.; Liu, D.; Yuan, K.; Yang, X.; Pan, W. Bioadhesive chitosan-loaded liposomes: A more efficient and higher permeable ocular delivery platform for timolol maleate. Int. J. Biol. Macromol. 2017, 94, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lancina, M. Fast Dissolving Dendrimer Nanofiber (DNF) Mats as Alternative to Eye Drops for More Efficient Topical Antiglaucoma Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 1861–1868. [Google Scholar] [CrossRef]
- Bin Sahadan, M.Y.; Tong, W.Y.; Tan, W.N.; Leong, C.R.; Bin Misri, M.N.; Chan, M.; Cheng, S.Y.; Shaharuddin, S. Phomopsidione nanoparticles coated contact lenses reduce microbial keratitis causing pathogens. Exp. Eye Res. 2019, 178, 10–14. [Google Scholar] [CrossRef]
- McAvoy, K.; Jones, D.; Thakur, R.R.S. Synthesis and Characterisation of Photocrosslinked poly(ethylene glycol) diacrylate Implants for Sustained Ocular Drug Delivery. Pharm. Res. 2018, 35, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Alexander-bryant, A.A.; Berg-foels, W.S. Vanden Berg-foels. In Bioengineering Strategies for Designing Targeted Cancer Therapies, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 118, ISBN 9780124071735. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Cao, X.; Qi, P. Therapeutic contact lenses for ophthalmic drug delivery: Major challenges. J. Biomater. Sci. Polym. Ed. 2020, 31, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Mahmoud, A.; Kamel, R. A Novel Method for Preparing Surface-Modified Fluocinolone Acetonide Loaded PLGA Nanoparticles for Ocular Use: In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2015, 17, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ameeduzzafar; Khanna, K.; Bhatnagar, A.; Ahmad, F.; Ali, A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 2018, 116, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Karasawa, K.; Onodera, R.; Takeuchi, H. Feasibility of drug delivery to the eye’s posterior segment by topical instillation of PLGA nanoparticles. Asian J. Pharm. Sci. 2017, 12, 394–399. [Google Scholar] [CrossRef]
- Eid, H.M.; Elkomy, M.H.; el Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 183. [Google Scholar] [CrossRef]
- Alkholief, M.; Albasit, H.; Alhowyan, A.; Alshehri, S.; Raish, M.; Kalam, A.; Alshamsan, A. Employing a PLGA-TPGS based nanoparticle to improve the ocular delivery of Acyclovir. Saudi Pharm. J. 2019, 27, 293–302. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen Biodegradable Nanoparticles: One Step Closer towards a Better Ocular Interaction Study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Dozois, M.D.; Chang, C.N.; Ahmad, A.; Ng, D.L.T.; Hileeto, D.; Liang, H.; Reyad, M.-M.; Boyd, S.; Jones, L.W.; et al. Prolonged Ocular Retention of Mucoadhesive Nanoparticle Eye Drop Formulation Enables Treatment of Eye Diseases Using Significantly Reduced Dosage. Mol. Pharm. 2016, 13, 2897–2905. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- FDA. “OZURDEX (Dexamethasone Intravitreal Implant)”, 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022315s009lbl.pdf (accessed on 15 June 2021).
- Akhter, S.; Ramazani, F.; Ahmad, M.Z.; Ahmad, F.; Rahman, Z.; Bhatnagar, A.; Storm, G. Ocular pharmacoscintigraphic and aqueous humoral drug availability of ganciclovir-loaded mucoadhesive nanoparticles in rabbits. Eur. J. Nanomed. 2013, 5. [Google Scholar] [CrossRef]
- Silva, B.; Marto, J.; Braz, B.S.; Delgado, E.; Almeida, A.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 576, 119020. [Google Scholar] [CrossRef]
- Bíró, T.; Aigner, Z. Current Approaches to Use Cyclodextrins and Mucoadhesive Polymers in Ocular Drug Delivery—A Mini-Review. Sci. Pharm. 2019, 87, 15. [Google Scholar] [CrossRef] [Green Version]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: Formulation development, in-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef]
- Pandit, J.; Sultana, Y.; Aqil, M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: Optimization, characterization, and in vitro toxicity evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1397–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chronopoulou, L.; Massimi, M.; Giardi, M.F.; Cametti, C.; Devirgiliis, L.C.; Dentini, M.; Palocci, C. Chitosan-coated PLGA nanoparticles: A sustained drug release strategy for cell cultures. Colloids Surf. B Biointerfaces 2013, 103, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Kundukad, B.; Somasundar, A.; Vijayan, S.; Khan, S.A.; Doyle, P.S. Design of Mucoadhesive PLGA Microparticles for Ocular Drug Delivery. ACS Appl. Bio Mater. 2018, 1, 561–571. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for ocular drug delivery: Modified and non-modified chitosan as a promising biocompatible carrier. Nanomedicine 2019, 14, 1889–1909. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Sherwin, T.; Patel, D.; McGhee, C.N.; Rupenthal, I.; Taylor, J.A.; Al-Kassas, R. Ex vivo and In vivo Evaluation of Chitosan Coated Nanostructured Lipid Carriers for Ocular Delivery of Acyclovir. Curr. Drug Deliv. 2016, 13, 923–934. [Google Scholar] [CrossRef]
- Kirch, J.; Schneider, M.; Abou, B.; Hopf, A.; Schaefer, U.F.; Schall, C.; Wagner, C.; Lehr, C.-M. Optical tweezers reveal relationship between microstructure and nanoparticle penetration of pulmonary mucus. Proc. Natl. Acad. Sci. USA 2012, 109, 18355–18360. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Gemeinhart, R.A. Understanding the adsorption mechanism of chitosan onto poly(lactide-co-glycolide) particles. Eur. J. Pharm. Biopharm. 2008, 70, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Billa, N.; Roberts, C.; Burley, J. Surfactant effects on the physical characteristics of Amphotericin B-containing nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 73–79. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Victor, R.D.S.; Santos, A.M.D.C.; de Sousa, B.V.; Neves, G.D.A.; Santana, L.N.D.L.; Menezes, R.R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- De Lima, I.A.; Khalil, N.M.; Tominaga, T.T.; Lechanteur, A.; Sarmento, B.; Mainardes, R.M. Mucoadhesive chitosan-coated PLGA nanoparticles for oral delivery of ferulic acid. Artif. Cells Nanomed. Biotechnol. 2018, 46, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feczkó, T.; Tóth, J.; Gyenis, J. Comparison of the preparation of PLGA–BSA nano- and microparticles by PVA, poloxamer and PVP. Colloids Surf. A Physicochem. Eng. Asp. 2008, 319, 188–195. [Google Scholar] [CrossRef]
- Mayol, L.; Serri, C.; Menale, C.; Crispi, S.; Piccolo, M.T.; Mita, L.; Giarra, S.; Forte, M.; Saija, A.; Biondi, M.; et al. Curcumin loaded PLGA–poloxamer blend nanoparticles induce cell cycle arrest in mesothelioma cells. Eur. J. Pharm. Biopharm. 2015, 93, 37–45. [Google Scholar] [CrossRef]
- Giarra, S.; Serri, C.; Russo, L.; Zeppetelli, S.; de Rosa, G.; Borzacchiello, A.; Biondi, M.; Ambrosio, L.; Mayol, L. Spontaneous arrangement of a tumor targeting hyaluronic acid shell on irinotecan loaded PLGA nanoparticles. Carbohydr. Polym. 2016, 140, 400–407. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; González, D.B.; Ortega-Vinuesa, J.L.; Alonso, M.J. Insulin-loaded PLGA nanoparticles for oral administration: An in vitro physico-chemical characterization. J. Biomed. Nanotechnol. 2009, 5, 45–53. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Lin, C.-C.; Hsieh, Y.-S.; Wu, Y.-T. Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach. Molecules 2021, 26, 3031. [Google Scholar] [CrossRef]
- Lakhani, P.; Patil, A.; Wu, K.-W.; Sweeney, C.; Tripathi, S.; Avula, B.; Taskar, P.; Khan, S.; Majumdar, S. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Int. J. Pharm. 2019, 572, 118771. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-L.; He, Y. Application of Box-Behnken designs in parameters optimization of differential pulse anodic stripping voltammetry for lead(II) determination in two electrolytes. Sci. Rep. 2017, 7, 2789. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Trabado, J.; López-García, A.; Martin-Pastor, M.; Diebold, Y.; Sanchez, A. Sorbitan ester nanoparticles (SENS) as a novel topical ocular drug delivery system: Design, optimization, and in vitro/ex vivo evaluation. Int. J. Pharm. 2018, 546, 20–30. [Google Scholar] [CrossRef]
- Madani, F.; Esnaashari, S.S.; Mujokoro, B.; Dorkoosh, F.; Khosravani, M.; Adabi, M. Investigation of Effective Parameters on Size of Paclitaxel Loaded PLGA Nanoparticles. Adv. Pharm. Bull. 2018, 8, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Canioni, R.; Reynaud, F.; Leite-Nascimento, T.; Gueutin, C.; Guiblin, N.; Ghermani, N.-E.; Jayat, C.; Daull, P.; Garrigue, J.-S.; Fattal, E.; et al. Tiny dexamethasone palmitate nanoparticles for intravitreal injection: Optimization and in vivo evaluation. Int. J. Pharm. 2021, 600, 120509. [Google Scholar] [CrossRef]
- Johnston, S.T.; Faria, M.; Crampin, E.J. An analytical approach for quantifying the influence of nanoparticle polydispersity on cellular delivered dose. J. R. Soc. Interface 2018, 15, 20180364. [Google Scholar] [CrossRef] [Green Version]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In Situ Gel of Triamcinolone Acetonide-Loaded Solid Lipid Nanoparticles for Improved Topical Ocular Delivery: Tear Kinetics and Ocular Disposition Studies. Nanomaterials 2018, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Schopf, L.R.; Popov, A.M.; Enlow, E.M.; Bourassa, J.L.; Ong, W.Z.; Nowak, P.; Chen, H. Topical Ocular Drug Delivery to the Back of the Eye by Mucus-Penetrating Particles. Transl. Vis. Sci. Technol. 2015, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Zhu, L.; Zhang, K.; Li, T.; Huang, S. Nanodelivery of triamcinolone acetonide with PLGA-chitosan nanoparticles for the treatment of ocular inflammation. Artif. Cells Nanomed. Biotechnol. 2021, 49, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019, 6, 191184. [Google Scholar] [CrossRef] [Green Version]
- Gathirwa, J.W.; Omwoyo, W.N.; Ogutu, B.; Oloo, F.; Swai, H.; Kalombo, L.; Melariri, P.; Maroa, G.; Mahanga, G.M. Preparation, characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int. J. Nanomed. 2014, 9, 3865–3874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbetta, C.D.C. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotech. 2012, 03, 208–225. [Google Scholar] [CrossRef]
- Kunasekaran, V.; Krishnamoorthy, K. Compatibility studies of rasagiline mesylate with selected excipients for an effective solid lipid nanoparticles formulation. Int. J. Pharm. Pharm. Sci. 2015, 7, 73–80. [Google Scholar]
- García-Millán, E.; Quintáns-Carballo, M.; Otero-Espinar, F.J. Solid-state characterization of triamcinolone acetonide nanosuspensiones by X-ray spectroscopy, ATR Fourier transforms infrared spectroscopy and differential scanning calorimetry analysis. Data Brief 2017, 15, 133–137. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhu, R.; Wang, Y.; Li, B.; Ma, Y.; Yin, Y. Synthesis and characterization of serial random and block-copolymers based on lactide and glycolide. Polym. Sci. Ser. B 2016, 58, 720–729. [Google Scholar] [CrossRef]
- Abou-ElNour, M.; Ishak, R.A.; Tiboni, M.; Bonacucina, G.; Cespi, M.; Casettari, L.; Soliman, M.E.; Geneidi, A.S. Triamcinolone acetonide-loaded PLA/PEG-PDL microparticles for effective intra-articular delivery: Synthesis, optimization, in vitro and in vivo evaluation. J. Control. Release 2019, 309, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Al Kayal, T.; Panetta, D.; Canciani, B.; Losi, P.; Tripodi, M.; Burchielli, S.; Ottoni, P.; Salvadori, P.A.; Soldani, G. Evaluation of the Effect of a Gamma Irradiated DBM-Pluronic F127 Composite on Bone Regeneration in Wistar Rat. PLoS ONE 2015, 10, e0125110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasmeen, S.; Kabiraz, M.K.; Saha, B.; Qadir; Gafur; Masum, S. Chromium (VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J. Plant Biotechnol. 2018, 45, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Dennis, G.; Harrison, W.; Agnes, K.; Erastus, G. Effect of Biological Control Antagonists Adsorbed on Chitosan Immobilized Silica Nanocomposite on Ralstonia solanacearum and Growth of Tomato Seedlings. Adv. Res. 2016, 6, 1–23. [Google Scholar] [CrossRef]
- Nagarwal, R.C.; Kant, S.; Singh, P.; Maiti, P.; Pandit, J. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, J.M.; Oh, I.J. Sustained Release of All-trans Retinoic Acid from Chitosan-coated Poly(DL-lactide-co-glycolide) Nanoparticles. Yakhak Hoeji 2019, 63, 367–373. [Google Scholar] [CrossRef]
- Yoncheva, K.; Vandervoort, J.; Ludwig, A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm. Dev. Technol. 2009, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Reardon, P.J.; Parhizkar, M.; Harker, A.H.; Browning, R.J.; Vassileva, V.; Stride, E.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C. Electrohydrodynamic fabrication of core–shell PLGA nanoparticles with controlled release of cisplatin for enhanced cancer treatment. Int. J. Nanomed. 2017, 12, 3913–3926. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Trinh, H.M.; Agrahari, V.; Sheng, Y.; Pal, D.; Mitra, A.K. Nanoparticle-Based Topical Ophthalmic Gel Formulation for Sustained Release of Hydrocortisone Butyrate. AAPS PharmSciTech 2015, 17, 294–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruschi, M.L. (Ed.) 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Steeples, L.R.; Jones, N.P.; Leal, I. Evaluating the Safety, Efficacy and Patient Acceptability of Intravitreal Fluocinolone Acetonide (0.2 mcg/Day) Implant in the Treatment of Non-Infectious Uveitis Affecting the Posterior Segment. Clin. Ophthalmol. 2021, 15, 1433–1442. [Google Scholar] [CrossRef]









| Emulsion Code | PLGA (mg/mL) | PF-127% (w/v) | Chitosan% (w/v) |
|---|---|---|---|
| CS-E1 | 4 | 2 | 2 |
| CS-E2 | 4.5 | 1.5 | 2 |
| CS-E3 | 3.5 | 1.5 | 2 |
| CS-E4 | 3.5 | 2 | 1.5 |
| CS-E5 | 4 | 1 | 1 |
| CS-E6 | 3.5 | 1.5 | 1 |
| CS-E7 | 4.5 | 1 | 1.5 |
| CS-E8 | 4.5 | 2 | 1.5 |
| CS-E9 | 4 | 1.5 | 1.5 |
| CS-E10 | 3.5 | 1 | 1.5 |
| CS-E11 | 4.5 | 1.5 | 1 |
| CS-E12 | 4 | 2 | 1 |
| CS-E13 | 4 | 1 | 2 |
| Emulsion Code | Stabilizer (% (w/v)) | Particle Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|---|
| A1 | PVA (0.25) | 359 ± 76.37 | −14 ± 5.44 | 0.15 ± 0.07 |
| CS-A1 | PVA (0.25) | 346 ± 125.37 | +18 ± 1.77 | 0.70 ± 0.53 |
| A2 | PF-127 (1) | 200 ± 61.16 | −20 ± 9.26 | 0.07 ± 0.01 |
| CS-A2 | PF-127 (1) | 187 ± 23.55 | +14 ± 5.44 | 0.06 ± 0.01 |
| Emulsion Code | Particle Size (nm) | PDI | Zeta Potential (mV) | %Encapsulation Efficiency |
|---|---|---|---|---|
| PE1 | 318.23 ± 18.61 | 0.220 ± 0.08 | −7.4 ± 2.43 | 64.80 ± 3.95 |
| CS-PE1 | 386.67 ± 15.14 | 0.136 ± 0.05 | +33.3 ± 4.69 | 57.14 ± 3.81 |
| PE2 | 240.47 ± 48.75 | 0.084 ± 0.05 | −6.9 ± 3.08 | 60.31± 2.46 |
| CS-PE2 | 351.33 ± 27.02 | 0.098 ± 0.04 | +31.97 ± 0.21 | 51.71 ± 1.82 |
| Frequency (cm−1) | Functional Group | Individual Components (cm−1) | Physical Mixture (cm−1) | E10-Nanoparticles (cm−1) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PLGA | PF-127 | TA | PLGA | PF-127 | TA | PLGA | PF-127 | TA | |||
| 3510 | OH | 3520 | 3495 | [68] | |||||||
| 3398 | OH | 3398 | 3398 | [69] | |||||||
| 3000–2950 | C–H | 2997–2954 | 2989–2953 | 2970–2889 | 2970–2886 | [68] | |||||
| 2891 | C–H | 2888 | 2889 | 2886 | [70] | ||||||
| 2985–2937 | C–H | 2989–2951 | 2970–2889 | 2970–2886 | [69] | ||||||
| 1705 | C=O | 1706 | 1762 | [69] | |||||||
| 1757 | C=O | 1759 | 1751 | 1762 | [68] | ||||||
| 1344 | O–H | 1343 | 1344 | 1344 | [70] | ||||||
| 1055 | C–F | 1057 | 1057 | 1060 | [67] | ||||||
| Formulation Code | Regression Coefficient (R2) | ||||
|---|---|---|---|---|---|
| Zero-Order | First-Order | Hixson–Crowell | Higuchi | Korsmeyer–Peppas | |
| PE 1 | 0.552 | 0.582 | 0.572 | 0.69 | 0.791 |
| PE 2 | 0.756 | 0.767 | 0.763 | 0.893 | 0.894 |
| E 10 | 0.338 | 0.41 | 0.386 | 0.464 | 0.621 |
| CS-PE1 | 0.237 | 0.252 | 0.247 | 0.387 | 0.641 |
| CS-PE2 | 0.503 | 0.541 | 0.528 | 0.676 | 0.845 |
| CS-E10 | 0.504 | 0.586 | 0.56 | 0.681 | 0.853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. https://doi.org/10.3390/pharmaceutics13101590
Dandamudi M, McLoughlin P, Behl G, Rani S, Coffey L, Chauhan A, Kent D, Fitzhenry L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics. 2021; 13(10):1590. https://doi.org/10.3390/pharmaceutics13101590
Chicago/Turabian StyleDandamudi, Madhuri, Peter McLoughlin, Gautam Behl, Sweta Rani, Lee Coffey, Anuj Chauhan, David Kent, and Laurence Fitzhenry. 2021. "Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery" Pharmaceutics 13, no. 10: 1590. https://doi.org/10.3390/pharmaceutics13101590