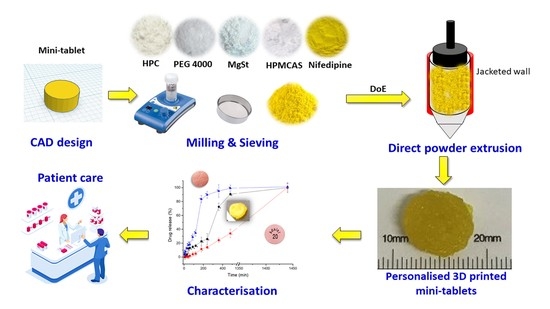
Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Quality by Design: Formulation Optimisation
2.2.2. Direct Powder Extrusion 3D Printing
Preparation of the Formulations
Geometry Design and 3D Printing Settings
2.2.3. Content Uniformity and Mass Uniformity
2.2.4. Imaging
2.2.5. Solid-State Characterisation
Fourier-Transform Infrared (FTIR) Spectroscopy
X-ray Powder Diffraction (pXRD)
Differential Scanning Calorimetry (DSC)
2.2.6. Dissolution Studies
Quantification of NFD by High Performance Liquid Chromatography (HPLC)
2.2.7. Data Processing and Statistical Analysis
3. Results
3.1. Design of Experiments (DoEs)
3.1.1. QbD-Based Model Development and Response Surface Analysis
3.1.2. Optimal Formulation and Validation of QbD
3.2. Content Uniformity and Mass Uniformity
3.3. Imaging
3.4. Solid-State Characterisation
3.4.1. PXRD
3.4.2. Differential Scanning Calorimetry (DSC)
3.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.5. Dissolution Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okafor-Muo, O.L.; Hassanin, H.; Kayyali, R.; ElShaer, A. 3D Printing of Solid Oral Dosage Forms: Numerous Challenges with Unique Opportunities. J. Pharm. Sci. 2020, 109, 3535–3550. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Brand, A.; Holgate, S.T.; Kristiansen, L.V.; Lehrach, H.; Palotie, A.; Prainsack, B. The future of technologies for personalised medicine. New Biotechnol. 2012, 29, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiboni, M.; Campana, R.; Frangipani, E.; Casettari, L. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int. J. Pharm. 2021, 596, 120290. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Terres, M.C.; Lalatsa, A. Applications of 3D printing in cancer. J. 3D Print. Med. 2018, 2, 115–128. [Google Scholar] [CrossRef]
- Konta, A.A.; Garcia-Pina, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Tyson, R.J.; Park, C.C.; Powell, J.R.; Patterson, J.H.; Weiner, D.; Watkins, P.B.; Gonzalez, D. Precision Dosing Priority Criteria: Drug, Disease, and Patient Population Variables. Front. Pharmacol. 2020, 11, 420. [Google Scholar] [CrossRef] [Green Version]
- Khaled, S.A.; Burley, J.; Alexander, M.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases 05/2017. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 6 June 2021).
- Brown, M.T.; Bussell, J.K. Medication adherence: WHO cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Klobusicky, J.J.; Aryasomayajula, A.; Marko, N. Evolving Patient Compliance Trends: Integrating Clinical, Insurance, and Extrapolated Socioeconomic Data. AMIA Annu. Symp. Proc. 2015, 2015, 766–774. [Google Scholar]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. Ficha Técnica Adalat Oros® 30 mg. Available online: https://cima.aemps.es/cima/publico/detalle.html?nregistro=59538 (accessed on 8 July 2021).
- U.S.P. <711> Dissolution; Rockville, MD, USA, 2021; Volume 41. Available online: www.usppf.com (accessed on 5 August 2021).
- U.S.P. Reagents: Test Solutions; Rockville, MD, USA, 2021; Volume 41. Available online: www.usppf.com (accessed on 5 August 2021).
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.R.; Danner, R.P.; Moser, J.D.; Chiu, S.W. Application of the Vrentas–Duda free-volume theory of diffusion below the glass-transition temperature: Application to hypromellose acetate succinate–solvent systems. J. Appl. Polym. Sci. 2019, 136, 47351. [Google Scholar] [CrossRef]
- Sarabu, S.; Kallakunta, V.R.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Zhang, F.; Repka, M.A. Hypromellose acetate succinate based amorphous solid dispersions via hot melt extrusion: Effect of drug physicochemical properties. Carbohydr. Polym. 2020, 233, 115828. [Google Scholar] [CrossRef] [PubMed]
- Picker-Freyer, K.M.; Durig, T. Physical mechanical and tablet formation properties of hydroxypropylcellulose: In pure form and in mixtures. AAPS PharmSciTech 2007, 8, E92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallakunta, V.R.; Sarabu, S.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Repka, M.A. Stable amorphous solid dispersions of fenofibrate using hot melt extrusion technology: Effect of formulation and process parameters for a low glass transition temperature drug. J. Drug Deliv. Sci. Technol. 2020, 58, 101395. [Google Scholar] [CrossRef] [PubMed]
- Emara, L.H.; Badr, R.M.; Elbary, A.A. Improving the dissolution and bioavailability of nifedipine using solid dispersions and solubilizers. Drug Dev. Ind. Pharm. 2002, 28, 795–807. [Google Scholar] [CrossRef]
- British Pharmacopeia. Dissolution. 2020. Available online: https://www.pharmacopoeia.com/the-british-pharmacopoeia (accessed on 5 August 2021).
- Liu, X.; Chen, D.; Zhang, R. Evaluation of monolithic osmotic tablet system for nifedipine delivery in vitro and in vivo. Drug Dev. Ind. Pharm. 2003, 29, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 72–74. [Google Scholar]
- Teixeira, A.Z.A. Hydroxypropylcellulose Controlled Release Tablet Matrix Prepared by Wet Granulation: Effect of Powder Properties and Polymer Composition. Braz. Arch. Biol. Technol. 2009, 52, 157–162. [Google Scholar] [CrossRef]
- Dürig, T.; Lusvardi, K.M.; Harcum, W.W. Hydroxypropylcellulose in Modified Release Matrix Systems: Polymer Molecular Weight Controls Drug Release Rated and Mechanism Ashland; Pharmaceutical Technology Report; PTR-029-01; Ashland: Wilmington, DE, USA, 2011; pp. 1–7. [Google Scholar]
- Ashland-KlucelTM Hydroxypropylcellulose—Physical and Chemical Properties. 2017. Available online: https://www.ashland.com/file_source/Ashland/Product/Documents/Pharmaceutical/PC_11229_Klucel_HPC.pdf (accessed on 8 August 2021).
- Roy, D.S.; Rohera, B.D. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur. J. Pharm. Sci. 2002, 16, 193–199. [Google Scholar] [CrossRef]






| Sample | Enthalpy of Fusion (J/g) | Melting (Onset) (°C) | Glass Transition (°C) |
|---|---|---|---|
| 3D printed mini-tablets | 7.9 ± 0.2 3.5 ± 0.4 | 51.3 ± 0.9 137.4 ± 0.6 | 49.7 ± 0.3 |
| Raw NFD | 110.8 ± 0.8 | 172.2 ± 1.0 | - |
| Physical Mixture | 28.41 ± 0.6 6.2 ± 0.8 | 53.2 ± 0.5 142.4 ± 0.9 | - |
| PEG 4000 | 191.9 ± 0.7 | 59.2 ± 1.0 | - |
| HPMCAS | - | - | 122.6 ± 0.7 |
| HPC | - | - | - |
| Sample | C=O (cm−1) | C-H (cm−1) |
|---|---|---|
| 3D printed mini-tablets | 1690 | 3333 |
| Raw NFD | 1682 | 3332 |
| Physical Mixture | 1680 | 3332 |
| PEG 4000 | N/A | N/A |
| HPMCAS | N/A | N/A |
| HPC | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Guirales, S.A.; Jurado, N.; Kara, A.; Lalatsa, A.; Serrano, D.R. Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets. Pharmaceutics 2021, 13, 1583. https://doi.org/10.3390/pharmaceutics13101583
Sánchez-Guirales SA, Jurado N, Kara A, Lalatsa A, Serrano DR. Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets. Pharmaceutics. 2021; 13(10):1583. https://doi.org/10.3390/pharmaceutics13101583
Chicago/Turabian StyleSánchez-Guirales, Sergio A., Noelia Jurado, Aytug Kara, Aikaterini Lalatsa, and Dolores R. Serrano. 2021. "Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets" Pharmaceutics 13, no. 10: 1583. https://doi.org/10.3390/pharmaceutics13101583