The Impact of Lipid Handling and Phase Distribution on the Acoustic Behavior of Microbubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
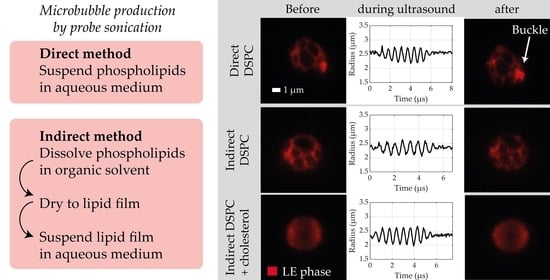
2.2. Microbubble Production
2.3. Physicochemical Characterization
2.4. Acoustical Characterization
2.5. Statistics
3. Results
3.1. Physicochemical Characterization
3.2. Acoustical Characterization
4. Discussion
4.1. Physicochemical Characterization
4.2. Acoustical Characterization
4.3. Lipid Phase Distribution and Acoustical Behavior
4.4. Implications of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Kosareva, A.; Abou-Elkacem, L.; Chowdhury, S.; Lindner, J.R.; Kaufmann, B.A. Seeing the Invisible—Ultrasound Molecular Imaging. Ultrasound Med. Biol. 2020, 46, 479–497. [Google Scholar] [CrossRef] [Green Version]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.G.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef]
- Versluis, M.; Stride, E.; Lajoinie, G.; Dollet, B.; Segers, T. Ultrasound Contrast Agent Modeling: A Review. Ultrasound Med. Biol. 2020, 46, 2117–2144. [Google Scholar] [CrossRef]
- Klibanov, A.L. Ultrasound Contrast Agents: Development of the Field and Current Status. In Contrast Agents II: Optical, Ultrasound, X-ray and Radiopharmaceutical Imaging; Krause, W., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2002; pp. 73–106. [Google Scholar]
- Van Rooij, T.; Daeichin, V.; Skachkov, I.; de Jong, N.; Kooiman, K. Targeted ultrasound contrast agents for ultrasound molecular imaging and therapy. Int. J. Hyperth. 2015, 31, 90–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overvelde, M.; Garbin, V.; Sijl, J.; Dollet, B.; de Jong, N.; Lohse, D.; Versluism, M. Nonlinear shell behavior of phospholipid-coated microbubbles. Ultrasound Med. Biol. 2010, 36, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Marmottant, P.; Meer, S.; Emmer, M.; Versluis, M.; Jong, N.; Hilgenfeldt, S.; Lohse, D. A model for large amplitude oscillations of coated bubbles accounting for buckling and rupture. J. Acoust. Soc. Am. 2005, 118, 3499–3505. [Google Scholar] [CrossRef] [Green Version]
- van Rooij, T.; Luan, Y.; Renaud, G.; van der Steen, A.F.; Versluis, M.; de Jong, N.; Kooiman, K. Non-linear response and viscoelastic properties of lipid-coated microbubbles: DSPC versus DPPC. Ultrasound Med. Biol. 2015, 41, 1432–1445. [Google Scholar] [CrossRef]
- Garg, S.; Thomas, A.A.; Borden, M.A. The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence. Biomaterials 2013, 34, 6862–6870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borden, M.A.; Pu, G.; Runner, G.J.; Longo, M.L. Surface phase behavior and microstructure of lipid/PEG-emulsifier monolayer-coated microbubbles. Colloids Surf. B Biointerfaces 2004, 35, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.A.; Martinez, G.V.; Ricker, J.; Tsvetkova, N.; Longo, M.; Gillies, R.J.; Dayton, P.A.; Ferrara, K.W. Lateral phase separation in lipid-coated microbubbles. Langmuir 2006, 22, 4291–4297. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.L.; Cherin, E.; Foster, F.S.; Goertz, D.E. Investigating the subharmonic response of individual phospholipid encapsulated microbubbles at high frequencies: A comparative study of five agents. Ultrasound Med. Biol. 2012, 38, 846–863. [Google Scholar] [CrossRef]
- Langeveld, S.A.G.; Schwieger, C.; Beekers, I.; Blaffert, J.; van Rooij, T.; Blume, A.; Kooiman, K. Ligand Distribution and Lipid Phase Behavior in Phospholipid-Coated Microbubbles and Monolayers. Langmuir 2020, 36, 3221–3233. [Google Scholar] [CrossRef]
- van Rooij, T.; Beekers, I.; Lattwein, K.R.; van der Steen, A.F.W.; de Jong, N.; Kooiman, K. Vibrational Responses of Bound and Nonbound Targeted Lipid-Coated Single Microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmer, M.; Vos, H.J.; Versluis, M.; de Jong, N. Radial modulation of single microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 2370–2379. [Google Scholar] [CrossRef]
- Van der Meer, S.M.; Dollet, B.; Voormolen, M.M.; Chin, C.T.; Bouakaz, A.; de Jong, N.; Versluism, M.; Lohse, D. Microbubble spectroscopy of ultrasound contrast agents. J. Acoust. Soc. Am. 2007, 121, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Lum, J.S.; Dove, J.D.; Murray, T.W.; Borden, M.A. Single Microbubble Measurements of Lipid Monolayer Viscoelastic Properties for Small-Amplitude Oscillations. Langmuir 2016, 32, 9410–9417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lum, J.S.; Stobbe, D.M.; Borden, M.A.; Murray, T.W. Photoacoustic technique to measure temperature effects on microbubble viscoelastic properties. Appl. Phys. Lett. 2018, 112, 111905. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Johnston, L.J. Phase evolution in cholesterol/DPPC monolayers: Atomic force microscopy and near field scanning optical microscopy studies. J. Microsc. 2002, 205, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, T.; Kato, S. Detailed Analysis of the Surface Area and Elasticity in the Saturated 1,2-Diacylphosphatidylcholine/Cholesterol Binary Monolayer System. Langmuir 2015, 31, 9086–9096. [Google Scholar] [CrossRef] [PubMed]
- Veatch, S.L.; Keller, S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Morris, R.; Bencsik, M.; Vangala, A.; Rades, T.; Perrie, Y. Development of a Novel Magnetic Resonance Imaging Contrast Agent for Pressure Measurements Using Lipid-Coated Microbubbles. J. Biomed. Nanotechnol. 2009, 5, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yoon, T.J.; Yoon, Y.I. Synthesis of ultrasound contrast agents: Characteristics and size distribution analysis (secondary publication). Ultrasonography 2017, 36, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Rasche, P.T.; Hughes, M.S.; Wojdyla, J.K.; Galen, K.P.; Wible, J.H.; Brandenburger, G.H. Detection of Individual Microbubbles of Ultrasound Contrast Agents. Investig. Radiol. 2004, 39, 187–195. [Google Scholar] [CrossRef]
- Chapman, D. Handbook of Lipid Research, Volume 4: The Physical Chemistry of Lipids. Biochem. Soc. Trans. 1987, 15, 184–185. [Google Scholar] [CrossRef]
- Kooiman, K.; Kokhuis, T.J.A.; van Rooij, T.; Skachkov, I.; Nigg, A.; Bosch, J.G.; van der Steen, A.F.W.; van Cappellen, W.A.; de Jong, N. DSPC or DPPC as main shell component influences ligand distribution and binding area of lipid-coated targeted microbubbles. Eur. J. Lipid Sci. Tech. 2014, 116, 1217–1227. [Google Scholar] [CrossRef]
- Hell, S.; Stelzer, E.H.K. Fundamental improvement of resolution with a 4Pi-confocal fluorescence microscope using two-photon excitation. Opt. Commun. 1992, 93, 277–282. [Google Scholar] [CrossRef]
- Beekers, I.; Lattwein, K.R.; Kouijzer, J.J.P.; Langeveld, S.A.G.; Vegter, M.; Beurskens, R.; Mastik, F.; Verduyn Lunel, R.; Verver, E.; van der Steen, A.F.W. Combined Confocal Microscope and Brandaris 128 Ultra-High-Speed Camera. Ultrasound Med. Biol. 2019, 45, 2575–2582. [Google Scholar] [CrossRef] [Green Version]
- Beekers, I.; van Rooij, T.; van der Steen, A.; Jong, N.; Verweij, M.; Kooiman, K. Acoustic Characterization of the CLINIcell for Ultrasound Contrast Agent Studies. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 66, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.T.; Lancée, C.; Borsboom, J.; Mastik, F.; Frijlink, M.E.; de Jong, N.; Versluis, M.; Lohse, D. Brandaris 128: A digital 25 million frames per second camera with 128 highly sensitive frames. Rev. Sci. Instrum. 2003, 74, 5026–5034. [Google Scholar] [CrossRef] [Green Version]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Ferrara, K.W.; Borden, M.A.; Zhang, H. Lipid-shelled vehicles: Engineering for ultrasound molecular imaging and drug delivery. Acc. Chem. Res. 2009, 42, 881–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirsi, S.; Borden, M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci. Eng. Technol. 2009, 1, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Yu, E.-S.; Ahn, D.J.; Ryu, Y.-S. Elasticity-Driven Membrane Budding through Cholesterol Concentration on Supported Lipid Monolayer–Bilayer Junction. Adv. Mater. Interfaces 2020, 7, 2000937. [Google Scholar] [CrossRef]
- Silvius, J.R. Thermotropic Phase Transitions of Pure Lipids in Model Membranes and Their Modifications by Membrane Proteins. In Lipid-Protein Interactions; John Wiley & Sons, Inc.: New York, NY, USA, 1982. [Google Scholar]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Influence of Cholesterol on the Phase Transition of Lipid Bilayers: A Temperature-Controlled Force Spectroscopy Study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef]
- Borden, M.A.; Kruse, D.E.; Caskey, C.F.; Shukui, Z.; Dayton, P.A.; Ferrara, K.W. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1992–2002. [Google Scholar] [CrossRef]
- Pu, G.; Longo, M.L.; Borden, M.A. Effect of Microstructure on Molecular Oxygen Permeation through Condensed Phospholipid Monolayers. J. Am. Chem. Soc. 2005, 127, 6524–6525. [Google Scholar] [CrossRef]
- Pu, G.; Borden, M.A.; Longo, M.L. Collapse and Shedding Transitions in Binary Lipid Monolayers Coating Microbubbles. Langmuir 2006, 22, 2993–2999. [Google Scholar] [CrossRef]
- Luan, Y.; Lajoinie, G.; Gelderblom, E.; Skachkov, I.; van der Steen, A.F.; Vos, H.J.; Versluis, M.; de Jong, N. Lipid shedding from single oscillating microbubbles. Ultrasound Med. Biol. 2014, 40, 1834–1846. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, K.; van Rooij, T.; Qin, B.; Mastik, F.; Vos, H.J.; Versluis, M.; Klibanov, A.L.; de Jong, N.; Villanueva, F.S.; Chen, X. Focal areas of increased lipid concentration on the coating of microbubbles during short tone-burst ultrasound insonification. PLoS ONE 2017, 12, e0180747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Costello, M.J.; Duncan, P.B.; Needham, D. Mechanical Properties and Microstructure of Polycrystalline Phospholipid Monolayer Shells: Novel Solid Microparticles. Langmuir 2003, 19, 8455–8466. [Google Scholar] [CrossRef]
- Browning, R.J.; Aron, M.; Booth, A.; Rademeyer, P.; Wing, S.; Brans, V.; Shrivastava, S.; Carugo, D.; Stride, E. Spectral Imaging for Microbubble Characterization. Langmuir 2020, 36, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, G.; Belloni, C.; Magnani, P.; Zito, F.; Pasini, A.; Sassi, I.; Meroni, M.; Mariani, M.; Vignali, M.; Siccardi, A.G.; et al. Two-step tumour targetting in ovarian cancer patients using biotinylated monoclonal antibodies and radioactive streptavidin. Eur. J. Nucl. Med. 1992, 19, 322–329. [Google Scholar] [CrossRef]












| MB Type | N | Shell Elasticity 1 (N/m) | Shell Viscosity 1 (×10−8 kg/s) | Max IQR of Oscillation Amplitude (%) | Median IQR of Oscillation Amplitude (%) |
|---|---|---|---|---|---|
| Direct DSPC | 44 | 0.14 (0.12–0.15) | 0.43 (0.38–0.61) | 8.0 | 1.5 |
| Indirect DSPC | 49 | 0.03 (0.01–0.06) | 0.99 (0.89–1.40) | 4.5 | 0.6 |
| DSPC-cholesterol 2 | 50 | 0.01 (0.01–0.02) | 1.39 (0.97–1.55) | 10.2 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langeveld, S.A.G.; Beekers, I.; Collado-Lara, G.; van der Steen, A.F.W.; de Jong, N.; Kooiman, K. The Impact of Lipid Handling and Phase Distribution on the Acoustic Behavior of Microbubbles. Pharmaceutics 2021, 13, 119. https://doi.org/10.3390/pharmaceutics13010119
Langeveld SAG, Beekers I, Collado-Lara G, van der Steen AFW, de Jong N, Kooiman K. The Impact of Lipid Handling and Phase Distribution on the Acoustic Behavior of Microbubbles. Pharmaceutics. 2021; 13(1):119. https://doi.org/10.3390/pharmaceutics13010119
Chicago/Turabian StyleLangeveld, Simone A.G., Inés Beekers, Gonzalo Collado-Lara, Antonius F. W. van der Steen, Nico de Jong, and Klazina Kooiman. 2021. "The Impact of Lipid Handling and Phase Distribution on the Acoustic Behavior of Microbubbles" Pharmaceutics 13, no. 1: 119. https://doi.org/10.3390/pharmaceutics13010119