Saturated Fatty Acid-Based In Situ Forming Matrices for Localized Antimicrobial Delivery
Abstract
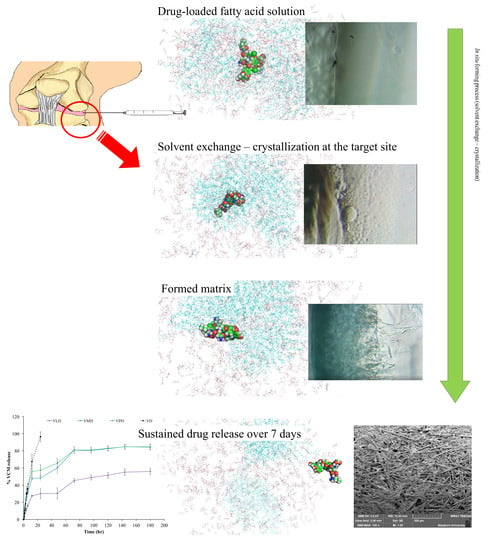
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of In Situ Forming Matrix
2.3. Evaluation of In Situ Forming Matrix Systems
2.3.1. pH, Density and Viscosity Evaluation
2.3.2. In Vitro Matrix Formation Behavior
2.3.3. Matrix Formation Rate Investigation (Cross-Sectional View)
2.3.4. Interfacial Phenomenon during the Initial Experimental Period
2.3.5. Electrical Potential Difference During Phase Transformation
2.3.6. Influence of KH2PO4 on Water Tolerance
2.3.7. Injectability Evaluation
2.3.8. In Vitro Drug Release
2.3.9. Aqueous Phase Influx Tracking
2.3.10. Topography of Transformed Matrices under Scanning Electron Microscopy (SEM)
2.3.11. Antimicrobial Activity Studies
2.3.12. Statistical Analysis
3. Results and Discussion
3.1. pH, Density, and Viscosity
3.2. In Vitro Matrix Formation
3.3. Matrix Formation Rate (Cross-Sectional View)
3.4. Interfacial Phenomenon during Initial Experimental Period
3.5. Electrical Potential Difference during Phase Transformation
3.6. Influence of KH2PO4 on Water Tolerance
3.7. Injectability
3.8. In Vitro Drug Release
3.9. Aqueous Phase Influx Tracking
3.10. Topography of Transformed Matrices
3.11. Antimicrobial Activity Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akilo, O.D.; Kumar, P.; Choonara, Y.E.; Du Toit, L.C.; Pradeep, P.; Modi, G.; Pillay, V. In situ thermo-co-electroresponsive mucogel for controlled release of bioactive agent. Int. J. Pharm. 2019, 559, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Messersmith, P.B. In situ forming biomaterials. Oral Maxillofac. Surg. Clin. N. Am. 2002, 14, 29–38. [Google Scholar] [CrossRef]
- Kubo, W.; Konno, Y.; Miyazaki, S.; Attwood, D. In Situ Gelling Pectin Formulations for Oral Sustained Delivery of Paracetamol. Drug Dev. Ind. Pharm. 2004, 30, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Mahadlek, J.; Tuntarawongsa, S. Peppermint oil/doxycycline hyclate-loaded Eudragit RS in situ forming gel for periodontitis treatment. J. Pharm. Investig. 2017, 48, 451–464. [Google Scholar] [CrossRef]
- Guo, J.-H.; Skinner, G.; Harcum, W.; Barnum, P. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharm. Sci. Technol. Today 1998, 1, 254–261. [Google Scholar] [CrossRef]
- Sheshala, R.; Hong, G.C.; Yee, W.P.; Meka, V.S.; Thakur, R.R.S. In situ forming phase-inversion implants for sustained ocular delivery of triamcinolone acetonide. Drug Deliv. Transl. Res. 2018, 9, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Phaechamud, T.; Senarat, S.; Puyathorn, N.; Praphanwittaya, P. Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and -microparticle. Int. J. Boil. Macromol. 2019, 135, 1261–1272. [Google Scholar] [CrossRef]
- Storozhylova, N.; Crecente-Campo, J.; Cabaleiro, D.; Lugo, L.; Dussouy, C.; Simões, S.; Monteiro, M.; Grandjean, C.; Alonso, M.J. An In Situ Hyaluronic Acid-Fibrin Hydrogel Containing Drug-Loaded Nanocapsules for Intra-Articular Treatment of Inflammatory Joint Diseases. Regen. Eng. Transl. Med. 2020, 6, 201–216. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Abdel-Salam, F.S.; Mahmoud, A.A.; Basalious, E.B.; Amer, M.S.; Mostafa, A.A.; Elkheshen, S.A. Long lasting in-situ forming implant loaded with raloxifene HCl: An injectable delivery system for treatment of bone injuries. Int. J. Pharm. 2019, 571, 118703. [Google Scholar] [CrossRef]
- Fogueri, L.R.; Singh, S. Smart polymers for controlled delivery of proteins and peptides: A review of patents. Recent Pat. Drug Deliv. Formul. 2009, 3, 40–48. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A.; Zhao, X.; Liu, X.; Wang, D.; Sun, F.; Li, Y. Design of a long-term antipsychotic in situ forming implant and its release control method and mechanism. Int. J. Pharm. 2012, 427, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Srichan, T.; Phaechamud, T. Designing Solvent Exchange-Induced In Situ Forming Gel from Aqueous Insoluble Polymers as Matrix Base for Periodontitis Treatment. AAPS Pharm. Sci. Tech. 2016, 18, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Setthajindalert, O. Cholesterol in situ forming gel loaded with doxycycline hyclate for intra-periodontal pocket delivery. Eur. J. Pharm. Sci. 2017, 99, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, T.; Zhu, Y.; Fu, Q.; Wu, W.; Deng, J.; Lan, L.; Shi, S. An in situ -forming phospholipid-based phase transition gel prolongs the duration of local anesthesia for ropivacaine with minimal toxicity. Acta Biomater. 2017, 58, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Beare-Rogers, J.L.; Dieffenbacher, A.; Holm, J.V. Lexicon of lipid nutrition (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 685–744. [Google Scholar] [CrossRef]
- Kingsbury, K.J.; Paul, S.; Crossley, A.; Morgan, D.M. The fatty acid composition of human depot fat. Biochem. J. 1961, 78, 541–550. [Google Scholar] [CrossRef]
- Elmadfa, I.; Kornsteiner, M. Fats and Fatty Acid Requirements for Adults. Ann. Nutr. Metab. 2009, 55, 56–75. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of myristic acid as a food ingredient. Food Chem. Toxicol. 2007, 45, 517–529. [Google Scholar] [CrossRef]
- Guse, C.; Koennings, S.; Maschke, A.; Hacker, M.; Becker, C.; Schreiner, S.; Blunk, T.; Spruss, T.; Göpferich, A. Biocompatibility and erosion behavior of implants made of triglycerides and blends with cholesterol and phospholipids. Int. J. Pharm. 2006, 314, 153–160. [Google Scholar] [CrossRef]
- White, B. Dietary fatty acids. Am. Fam. Physician 2009, 80, 345–350. [Google Scholar]
- Anneken, D.J.; Both, S.; Christoph, R.; Fieg, G.; Steinberner, U.; Westfechtel, A. Fatty acids. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2006; Volume 14, pp. 74–116. [Google Scholar]
- Vervaeck, A.; Saerens, L.; De Geest, B.; De Beer, T.; Carleer, R.; Adriaensens, P.; Remon, J.; Vervaet, C. Prilling of fatty acids as a continuous process for the development of controlled release multiparticulate dosage forms. Eur. J. Pharm. Biopharm. 2013, 85, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervaeck, A.; Monteyne, T.; Siepmann, F.; Boone, M.; Van Hoorebeke, L.; De Beer, T.; Siepmann, J.; Remon, J.P.; Vervaet, C. Fatty acids for controlled release applications: A comparison between prilling and solid lipid extrusion as manufacturing techniques. Eur. J. Pharm. Biopharm. 2015, 97, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Joy, P. Influence of chain length of long-chain fatty acid surfactant on the thermal conductivity of magnetite nanofluids in a magnetic field. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 555, 525–531. [Google Scholar] [CrossRef]
- Livermore, D.M. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents 2000, 16, 3–10. [Google Scholar] [CrossRef]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef] [Green Version]
- Engemann, J.J.; Carmeli, Y.; Cosgrove, S.E.; Jr, V.G.F.; Bronstein, M.Z.; Trivette, S.L.; Briggs, J.P.; Sexton, D.J.; Kaye, K.S. Adverse Clinical and Economic Outcomes Attributable to Methicillin Resistance among Patients withStaphylococcus aureusSurgical Site Infection. Clin. Infect. Dis. 2003, 36, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Watanakunakorn, C. Mode of action and in-vitro activity of vancomycin. J. Antimicrob. Chemother. 1984, 14, 7–18. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Khan, I.A.; Zhang, J.; Melek, F.R.; El-Hawary, S.S.; Jacob, M.R.; Muhammad, I. Methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecalis and Enterococcus faecium active dimeric isobutyrylphloroglucinol from Ivesia gordonii. Nat. Prod. Commun. 2014, 9, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Dunne, M.W.; Sahm, D.; Puttagunta, S. Use of vancomycin as a surrogate for dalbavancin in vitro susceptibility testing: Results from the DISCOVER studies. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Guillamet, C.V.; Kollef, M.H. Treatment of Gram-positive infections in critically ill patients. BMC Infect. Dis. 2014, 14, 92. [Google Scholar] [CrossRef] [Green Version]
- Micromedex. Vancomycin; Thomson Micromedex: Greenwood Village, CO, USA, 2017. [Google Scholar]
- Roy, M.E.; Peppers, M.P.; Whiteside, L.A.; LaZear, R.M. Vancomycin Concentration in Synovial Fluid: Direct Injection into the Knee vs. Intravenous Infusion. J. Arthroplast. 2014, 29, 564–568. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Bakhshi, H.; Ecker, N.U.; Parvizi, J.; Gehrke, T.; Kendoff, D. Organism Profile in Periprosthetic Joint Infection: Pathogens Differ at Two Arthroplasty Infection Referral Centers in Europe and in the United States. J. Knee Surg. 2014, 27, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerke-Kroll, B.T.; Christ, A.B.; McLawhorn, A.S.; Sculco, P.; Jules-Elysée, K.M.; Sculco, T.P. Periprosthetic Joint Infections Treated with Two-Stage Revision over 14Years: An Evolving Microbiology Profile. J. Arthroplast. 2014, 29, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, P.H. Methicillin-resistant staphylococcus aureus infections of the eye and orbit (an american ophthalmological society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 322–345. [Google Scholar] [PubMed]
- Sfeir, M.M.; Obeid, Y.; Eid, C.; Saliby, M.; Farra, A.; Farhat, H.; Mokhbat, J.E. Prevalence of Staphylococcus aureus methicillin-sensitive and methicillin-resistant nasal and pharyngeal colonization in outpatients in Lebanon. Am. J. Infect. Control 2014, 42, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, M.; Widmer, A.F.; Dangel, M.; Filippi, A.; Frei, R.; Meyer, J. Methicillin-resistant Staphylococcus aureus (MRSA) among dental patients: A problem for infection control in dentistry? Clin. Oral Investig. 2008, 13, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Elward, A.M.; McAndrews, J.M.; Young, V.L. Methicillin-Sensitive and Methicillin-Resistant Staphylococcus aureus: Preventing Surgical Site Infections Following Plastic Surgery. Aesthetic Surg. J. 2009, 29, 232–244. [Google Scholar] [CrossRef]
- Stevens, C.M.; Tetsworth, K.D.; Calhoun, J.H.; Mader, J.T. An articulated antibiotic spacer used for infected total knee arthroplasty: A comparative in vitro elution study of Simplex® and Palacos® bone cements. J. Orthop. Res. 2005, 23, 27–33. [Google Scholar] [CrossRef]
- Chantadee, T.; Sawangsri, P.; Santimaleeworagun, W.; Phaechamud, T. Vancomycin hydrochloride-loaded stearic acid/lauric acid in situ forming matrix for antimicrobial inhibition in patients with joint infection after total knee arthroplasty. Mater. Sci. Eng. C 2020, 115, 110761. [Google Scholar] [CrossRef]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Vancomycin HCl-loaded lauric acid in situ-forming gel with phase inversion for periodontitis treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101615. [Google Scholar] [CrossRef]
- MicroMath. MicroMath Scientist Handbook Rev; 7EEF. MicroMath: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Costa, P.C.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Chanyaboonsub, N.; Setthajindalert, O. Doxycycline hyclate-loaded bleached shellac in situ forming microparticle for intraperiodontal pocket local delivery. Eur. J. Pharm. Sci. 2016, 93, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Stainsby, G.; Alexander, A.E. Studies of soap solutions. Part I. The fatty acid soaps and their hydrolysis in aqueous solutions. Trans. Faraday Soc. 1949, 45, 585. [Google Scholar] [CrossRef]
- Mukerjee, P.; Mysels, K.J.; Dulin, C.I. Dilute Solutions of Amphipathic Ions. I. Conductivity of Strong Salts and Dimerization. J. Phys. Chem. 1958, 62, 1390–1396. [Google Scholar] [CrossRef]
- Craven, D.M.S.B.M. Physical Properties of Fatty Acids and their Extracellular and Intracellular Distribution. In Polyunsaturated Fatty Acids in Human Nutrition; Workshop, N.N., Ed.; Nestec Ltd.; Vevey/Raven Press Ltd.: New York, NY, USA, 1992; Volume 28, pp. 25–39. [Google Scholar]
- Eiteman, M.A.; Goodrum, J.W. Density and viscosity of low-molecular weight triglycerides and their mixtures. J. Am. Oil Chem. Soc. 1994, 71, 1261–1265. [Google Scholar] [CrossRef]
- The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals; O’Neil, M.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; p. 2708. [Google Scholar]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Fatty acids and related phase change materials for reliable thermal energy storage at moderate temperatures. Sol. Energy Mater. Sol. Cells 2017, 167, 109–120. [Google Scholar] [CrossRef]
- Rodenbush, C.M.; Hsieh, F.H.; Viswanath, D.S. Density and viscosity of vegetable oils. J. Am. Oil Chem. Soc. 1999, 76, 1415–1419. [Google Scholar] [CrossRef]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T.; Jantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J. Pharm. Sci. 2017, 13, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, M.; Takebayashi, S.; Taguchi, M.; Kasahara, Y.; Minami, H.; Matsuzawa, H. Dynamical dimer structure and liquid structure of fatty acids in their binary liquid mixture: Decanoic/octadecanoic acid and decanoic/dodecanoic acid systems. Chem. Phys. Lipids 2005, 133, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Raphaelides, S.N.; Georgiadis, N. Effect of fatty acids on the rheological behaviour of amylomaize starch dispersions during heating. Food Res. Int. 2008, 41, 75–88. [Google Scholar] [CrossRef]
- Meng, S.; Ma, Y.; Sun, D.-W.; Wang, L.; Liu, T. Properties of starch-palmitic acid complexes prepared by high pressure homogenization. J. Cereal Sci. 2014, 59, 25–32. [Google Scholar] [CrossRef]
- Small, N.M. The Physical Chemistry of Lipids. In The Physical Chemistry of Lipids; Springer Science and Business Media LLC: New York, NY, USA, 1986; Volume 4, p. 264. [Google Scholar]
- Viken, A.L.; Spildo, K.; Reichenbach-Klinke, R.; Djurhuus, K.; Skauge, T. Influence of Weak Hydrophobic Interactions on in Situ Viscosity of a Hydrophobically Modified Water-Soluble Polymer. Energy Fuels 2017, 32, 89–98. [Google Scholar] [CrossRef]
- Liang, Y.; Yuan, X.; Wang, L.; Zhou, X.; Ren, X.; Huang, Y.; Zhang, M.; Wu, J.; Wen, W. Highly stable and efficient electrorheological suspensions with hydrophobic interaction. J. Colloid Interface Sci. 2020, 564, 381–391. [Google Scholar] [CrossRef]
- Yamamoto, S. Drying of gelled sugar solutions—Water diffusion behavior. Chem. Eng. J. 2002, 86, 179–184. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.; Stapley, A.G. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Cumming, H.; Rücker, C. Octanol-Water Partition Coefficient Measurement by a Simple 1H NMR Method. ACS Omega 2017, 2, 6244–6249. [Google Scholar] [CrossRef]
- Sasaki, H.; Kojima, M.; Mori, Y.; Nakamura, J.; Shibasaki, J. Enhancing effect of pyrrolidone derivatives on transdermal drug delivery. I. Int. J. Pharm. 1988, 44, 15–24. [Google Scholar] [CrossRef]
- Ovrutsky, A.; Prokhoda, A.; Rasshchupkyna, M. Basic Concepts of Theory of Phase Transformations. In Computational Materials Science; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 35–69. [Google Scholar]
- Kakran, M.; Sahoo, N.G.; Tan, I.-L.; Li, L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J. Nanopart. Res. 2012, 14, 757. [Google Scholar] [CrossRef]
- Pimienta, V.; Lavabre, D.; Buhse, T.; Micheau, J.-C. Correlation between Electric Potential and Interfacial Tension Oscillations in a Water−Oil−Water System. J. Phys. Chem. B 2004, 108, 7331–7336. [Google Scholar] [CrossRef]
- Dos Santos, A.P.; Levin, Y. Surface and interfacial tensions of Hofmeister electrolytes. Faraday Discuss. 2013, 160, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Roger, K.; Cabane, B. Why Are Hydrophobic/Water Interfaces Negatively Charged? Angew. Chem. Int. Ed. 2012, 51, 5625–5628. [Google Scholar] [CrossRef]
- Törnquist, M.; Michaels, T.C.T.; Sanagavarapu, K.; Yang, X.; Meisl, G.; Cohen, S.I.A.; Knowles, T.P.J.; Linse, S. Secondary nucleation in amyloid formation. Chem. Commun. 2018, 54, 8667–8684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashkovskaya, A.A.; Vazdar, M.; Zimmermann, L.; Jovanovic, O.; Pohl, P.; Pohl, E.E. Mechanism of Long-Chain Free Fatty Acid Protonation at the Membrane-Water Interface. Biophys. J. 2018, 114, 2142–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moelbert, S.; Normand, B.; Rios, P.D.L. Kosmotropes and chaotropes: Modelling preferential exclusion, binding and aggregate stability. Biophys. Chem. 2004, 112, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rungseevijitprapa, W.; Bodmeier, R. Injectability of biodegradable in situ forming microparticle systems (ISM). Eur. J. Pharm. Sci. 2009, 36, 524–531. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yang, M.; Jia, D.; Wang, Y.; Li, B.; Hou, Y.; Zhang, Y.; Wu, Q. Experimental evaluation of cooling performance by friction coefficient and specific friction energy in nanofluid minimum quantity lubrication grinding with different types of vegetable oil. J. Clean. Prod. 2016, 139, 685–705. [Google Scholar] [CrossRef]
- Fakhari, A.; Phan, Q.; Thakkar, S.V.; Middaugh, C.R.; Berkland, C.J. Hyaluronic Acid Nanoparticles Titrate the Viscoelastic Properties of Viscosupplements. Langmuir 2013, 29, 5123–5131. [Google Scholar] [CrossRef]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; El-Refaie, W.M.; El-Massik, M.A.; Abdallah, O.Y. Lecithin-based nanostructured gels for skin delivery: An update on state of art and recent applications. J. Control. Release 2014, 180, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Mahadlek, J.; Chuenbarn, T. In situ forming gel comprising bleached shellac loaded with antimicrobial drugs for periodontitis treatment. Mater. Des. 2016, 89, 294–303. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Thomas, N.L.; Windle, A. A theory of case II diffusion. Polymers 1982, 23, 529–542. [Google Scholar] [CrossRef]
- Padmaa, M.P.; Preethy, A.J.; Setty, C.M.; Christopher, G.V.P. Release kinetics—Concepts and applications. IJPRT 2018, 8, 12. [Google Scholar]
- Guinan, J.; Wang, S.; Hazbun, T.R.; Yadav, H.; Thangamani, S. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Umerska, A.; Cassisa, V.; Matougui, N.; Joly-Guillou, M.-L.; Eveillard, M.; Saulnier, P. Antibacterial action of lipid nanocapsules containing fatty acids or monoglycerides as co-surfactants. Eur. J. Pharm. Biopharm. 2016, 108, 100–110. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, J.; De Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B: Boil. 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morlière, C.; Bolzinger-Thevenin, M.A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, L.; Zhang, C.; Liu, D.; Meng, S.; Zhang, W.; Meng, S. Effect of Polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure. Pharmaceutics 2019, 11, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, T.A.; Ibrahim, H.M.; Samy, A.M.; Kaseem, A.; Nutan, M.T.H.; Hussain, M.D. Biodegradable Injectable In Situ Implants and Microparticles for Sustained Release of Montelukast: In Vitro Release, Pharmacokinetics, and Stability. AAPS Pharm. Sci. Tech. 2014, 15, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Takayama, K.; Kawakami, Y.; Kobayashi, M.; Greco, N.; Cummins, J.H.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res. 2014, 16, 482. [Google Scholar] [CrossRef] [Green Version]
- Micromedex. Dimethyl Sulfoxide; Thomson Micromedex: Greenwood Village, CO, USA, 2020. [Google Scholar]
- Elmoazzen, H.Y.; Poovadan, A.; Law, G.; Elliott, J.A.W.; McGann, L.E.; Jomha, N. Dimethyl sulfoxide toxicity kinetics in intact articular cartilage. Cell Tissue Bank. 2006, 8, 125–133. [Google Scholar] [CrossRef]
- Whiteside, L.A.; Roy, M.E.; Nayfeh, T.A.; Surgeon, O. Research Director Intra-articular infusion. Bone Jt. J. 2016, 98-B, 31–36. [Google Scholar] [CrossRef] [Green Version]








| Formulation Code | Fatty Acid | VCM (1 g) | Solvent | |
|---|---|---|---|---|
| Type | Amount (g) | (Adjust to 100 mL) | ||
| 35CPLD | CPL | 35.06 | - | DMSO |
| 35CPRD | CPR | 35.06 | - | DMSO |
| 35LD | LAU | 35.06 | - | DMSO |
| 35MD | MYR | 35.06 | - | DMSO |
| 35PD | PAL | 35.06 | - | DMSO |
| 35SD | STR | 35.06 | - | DMSO |
| 1.75CPLD | CPL | 25.24 | - | DMSO |
| 1.75CPRD | CPR | 30.15 | - | DMSO |
| 1.75LD | LAU | 35.06 | - | DMSO |
| 1.75MD | MYR | 39.97 | - | DMSO |
| 1.75PD | PAL | 44.88 | - | DMSO |
| 1.75SD | STR | 49.78 | - | DMSO |
| 35CPLN | CPL | 35.06 | - | NMP |
| 35CPRN | CPR | 35.06 | - | NMP |
| 35LN | LAU | 35.06 | - | NMP |
| 35MN | MYR | 35.06 | - | NMP |
| 35PN | PAL | 35.06 | - | NMP |
| 35SN | STR | 35.06 | - | NMP |
| 1.75CPLN | CPL | 25.24 | - | NMP |
| 1.75CPRN | CPR | 30.15 | - | NMP |
| 1.75LN | LAU | 35.06 | - | NMP |
| 1.75MN | MYR | 39.97 | - | NMP |
| 1.75PN | PAL | 44.88 | - | NMP |
| 1.75SN | STR | 49.78 | - | NMP |
| VD | - | - | 1.00 | DMSO |
| VCPLD | CPL | 35.06 | 1.00 | DMSO |
| VCPRD | CPR | 35.06 | 1.00 | DMSO |
| VLD | LAU | 35.06 | 1.00 | DMSO |
| VMD | MYR | 35.06 | 1.00 | DMSO |
| VPD | PAL | 35.06 | 1.00 | DMSO |
| Formulation | pH | Density (g/cm−3) | Viscosity (cPs) |
|---|---|---|---|
| 35CPLD | 5.55 ± 0.07 | 1.0240 ± 0.0002 | 6.29 ± 0.04 |
| 35CPRD | 6.13 ± 0.02 | 1.0205 ± 0.0001 | 7.51 ± 0.02 |
| 35LD | 6.05 ± 0.11 | 1.0161 ± 0.0002 | 8.05 ± 0.05 |
| 35MD | 6.19 ± 0.11 | 1.0142 ± 0.0000 | 9.54 ± 0.17 |
| 35PD | 6.22 ± 0.05 | 1.0119 ± 0.0001 | 10.90 ± 0.05 |
| 35SD | 6.78 ± 0.01 | 1.0109 ± 0.0001 | ND |
| 1.75CPLD | 5.97 ± 0.02 | 1.0407 ± 0.0001 | 5.47 ± 0.12 |
| 1.75CPRD | 6.44 ± 0.04 | 1.0345 ± 0.0001 | 6.08 ± 0.05 |
| 1.75LD | 6.15 ± 0.03 | 1.0154 ± 0.0001 | 7.95 ± 0.10 |
| 1.75MD | 5.95 ± 0.03 | 1.0026 ± 0.0002 | 11.86 ± 0.08 |
| 1.75PD | 5.98 ± 0.04 | 0.9899 ± 0.0002 | ND |
| 1.75SD | 6.28 ± 0.01 | 0.9799 ± 0.0002 | ND |
| 35CPLN | 5.35 ± 0.01 | 0.9894 ± 0.0001 | 7.07 ± 0.20 |
| 35CPRN | 5.76 ± 0.05 | 0.9825 ± 0.0002 | 7.96 ± 0.29 |
| 35LN | 5.93 ± 0.03 | 0.9797 ± 0.0001 | 8.43 ± 0.09 |
| 35MN | 5.98 ± 0.01 | 0.9767 ± 0.0001 | 8.91 ± 0.40 |
| 35PN | 6.06 ± 0.01 | 0.9731 ± 0.0001 | 8.92 ± 0.54 |
| 35SN | 6.00 ± 0.00 | 0.9759 ± 0.0001 | 9.24 ± 0.44 |
| 1.75CPLN | 6.53 ± 0.05 | 0.9935 ± 0.0001 | 5.63 ± 0.06 |
| 1.75CPRN | 6.53 ± 0.05 | 0.9947 ± 0.0000 | 5.73 ± 0.08 |
| 1.75LN | 5.81 ± 0.04 | 0.9775 ± 0.0000 | 7.57 ± 0.16 |
| 1.75MN | 5.60 ± 0.01 | 0.9664 ± 0.0001 | 9.49 ± 0.20 |
| 1.75PN | 5.41 ± 0.05 | 0.9579 ± 0.0001 | 10.90 ± 0.20 |
| 1.75SN | 5.16 ± 0.01 | 0.9529 ± 0.0003 | 13.97 ± 0.05 |
| Formula | Zero-Order | First-Order | Higuchi’s | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| r2 | k | r2 | k | r2 | k | r2 | k | n | |
| VLD | 0.8374 | 0.3113 | 0.5863 | 0.0056 | 0.9584 | 4.4057 | 0.9593 | 0.0498 | 0.5062 |
| VMD | 0.8130 | 0.7792 | 0.5470 | 0.0088 | 0.9451 | 8.3685 | 0.9334 | 0.1048 | 0.4923 |
| VPD | 0.7537 | 0.7772 | 0.5576 | 0.0082 | 0.9169 | 8.5386 | 0.9471 | 0.1239 | 0.4589 |
| Formula | Clear Zone Diameter (mean ± SD) | ||||
|---|---|---|---|---|---|
| S. aureus ATCC 25923 | S. aureus ATCC 43300 | S. aureus DMST 6532 | E. coli ATCC 8739 | C. albicans ATCC 17100 | |
| 35CPLD | 11.00 ± 0.00 | 10.00 ± 0.00 | 10.00 ± 0.00 | 10.33 ± 0.58 | 40.33 ± 2.52 |
| 35CPRD | 7.67 ± 0.58 | 7.00 ± 0.00 | 7.00 ± 0.00 | - | 24.33 ± 0.58 |
| 35LD | 7.33 ± 0.58 | 8.33 ± 2.31 | 10.00 ± 0.00 | - | 16.33 ± 0.58 |
| 35MD | 7.00 ± 0.00 | 7.00 ± 0.00 | 7.00 ± 0.00 | - | 16.67 ± 1.53 |
| 35PD | 7.00 ± 0.00 | 7.00 ± 0.00 | 7.00 ± 0.00 | - | 13.67 ± 1.53 |
| VCPLD | 30.33 ± 1.53 | 30.00 ± 0.00 | 27.33 ± 2.08 | 17.33 ± 0.58 | 39.33 ± 1.15 |
| VCPRD | 31.67 ± 2.08 | 29.67 ± 0.58 | 28.00 ± 2.00 | 16.33 ± 0.58 | 28.00 ± 2.00 |
| VLD | 28.00 ± 3.61 | 28.00 ± 1.00 | 34.67 ± 3.06 | 16.67 ± 0.58 | 16.67 ± 0.58 |
| VMD | 27.00 ± 1.73 | 27.00 ± 0.00 | 31.67 ± 2.08 | 15.33 ± 1.53 | 15.33 ± 1.15 |
| VPD | 26.33 ± 1.53 | 27.67 ± 1.53 | 26.67 ± 0.58 | 13.00 ± 1.00 | 14.33 ± 0.58 |
| VD | 27.33 ± 0.58 | 29.67 ± 0.58 | 28.33 ± 1.15 | 16.67 ± 0.58 | 18.33 ± 0.58 |
| DMSO | 7.67 ± 1.15 | 6.67 ± 0.58 | 7.00 ± 0.00 | 13.00 ± 1.00 | 19.67 ± 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Phaechamud, T. Saturated Fatty Acid-Based In Situ Forming Matrices for Localized Antimicrobial Delivery. Pharmaceutics 2020, 12, 808. https://doi.org/10.3390/pharmaceutics12090808
Chantadee T, Santimaleeworagun W, Phorom Y, Phaechamud T. Saturated Fatty Acid-Based In Situ Forming Matrices for Localized Antimicrobial Delivery. Pharmaceutics. 2020; 12(9):808. https://doi.org/10.3390/pharmaceutics12090808
Chicago/Turabian StyleChantadee, Takron, Wichai Santimaleeworagun, Yaowaruk Phorom, and Thawatchai Phaechamud. 2020. "Saturated Fatty Acid-Based In Situ Forming Matrices for Localized Antimicrobial Delivery" Pharmaceutics 12, no. 9: 808. https://doi.org/10.3390/pharmaceutics12090808