Synthesis and Ex Vivo Trans-Corneal Permeation of Penetratin Analogues as Ophthalmic Carriers: Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microwave Peptide Synthesis
2.3. Trans-Corneal Permeation Studies
2.4. Analytical Method
2.5. Circular Dichroism (CD) Measurements
2.6. Data Processing
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclosures
References
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Santini, A.; Ferreira, J.; Sánchez-López, E.; Ettcheto, M.; Cano, A.; Camins, A.; Espina, M.; García, M.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef]
- Pescina, S.; Ostacolo, C.; Gomez-Monterrey, I.; Sala, M.; Bertamino, A.; Sonvico, F.; Padula, C.; Santi, P.; Bianchera, A.; Nicoli, S. Cell penetrating peptides in ocular drug delivery: State of the art. J. Control. Release 2018, 284, 84–102. [Google Scholar] [CrossRef]
- Pescina, S.; Sala, M.; Padula, C.; Scala, M.C.; Spensiero, A.; Belletti, S.; Gatti, R.; Novellino, E.; Campiglia, P.; Santi, P.; et al. Design and Synthesis of New Cell Penetrating Peptides: Diffusion and Distribution Inside the Cornea. Mol. Pharm. 2016, 13, 3876–3883. [Google Scholar] [CrossRef] [Green Version]
- Ambroggio, E.E.; Caruso, B.; Villarreal, M.A.; Raussens, V.; Fidelio, G.D. Reversing the peptide sequence impacts on molecular surface behaviour. Colloids Surf. B Biointerfaces 2016, 139, 25–32. [Google Scholar] [CrossRef]
- Deshayes, S.; Morris, M.C.; Divita, G.; Heitz, F. Interactions of Primary Amphipathic Cell Penetrating Peptides with Model Membranes: Consequences on the Mechanisms of Intracellular Delivery of Therapeutics. Curr. Pharm. Des. 2005, 11, 3629–3638. [Google Scholar] [CrossRef]
- Deshayes, S.; Morris, M.C.; Divita, G.; Heitz, F. Interactions of amphipathic CPPs with model membranes. Biochim. et Biophys. Acta (BBA) Biomembr. 2006, 1758, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Fischer, P.M.; Zhelev, N.; Wang, S.; Melville, J.; Fåhraeus, R.; Lane, D.P. Structure-activity relationship of truncated and substituted analogues of the intracellular delivery vector Penetratin. J. Pept. Res. 2000, 55, 163–172. [Google Scholar] [CrossRef]
- Pescina, S.; Govoni, P.; Potenza, A.; Padula, C.; Santi, P.; Nicoli, S. Development of a Convenient ex vivo Model for the Study of the Transcorneal Permeation of Drugs: Histological and Permeability Evaluation. J. Pharm. Sci. 2015, 104, 63–71. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Khan, A.R.; Fu, M.; Wang, R.; Ji, J.; Zhai, G. Cell-penetrating peptide: A means of breaking through the physiological barriers of different tissues and organs. J. Control. Release 2019, 309, 106–124. [Google Scholar] [CrossRef]
- Liu, C.; Tai, L.; Zhang, W.; Wei, G.; Pan, W.; Lu, W. Penetratin, a potentially powerful absorption enhancer for noninvasive intraocular drug delivery. Mol. Pharm. 2014, 11, 1218–1227. [Google Scholar] [CrossRef]
- Jiang, K.; Gao, X.; Shen, Q.; Zhan, C.; Zhang, Y.; Xie, C.; Wei, G.; Lu, W. Discerning the composition of penetratin for safe penetration from cornea to retina. Acta Biomater. 2017, 63, 123–134. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Li, L.; Han, M.; Tang, S.; Wang, T.; Han, J.; He, X.; He, X.; Wang, A.; et al. A novel dendrimer-based complex co-modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019, 26, 989–1001. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.H.; Ammar, D.A.; Petrash, M. Cell Penetration Peptides for Enhanced Entry of αB-Crystallin into Lens Cells. Investig. Opthalmology Vis. Sci. 2013, 54, 2–8. [Google Scholar] [CrossRef] [Green Version]
- García, M.L.; Pérez, Y.; Gómara, M.J.; Vasconcelos, A.; Vega, E.; Haro, I. Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery. Int. J. Nanomed. 2015, 10, 609–631. [Google Scholar] [CrossRef] [Green Version]
- Kalafatovic, D.; Giralt, E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22, 1929. [Google Scholar] [CrossRef] [Green Version]
- Henriques, S.T.; Castanho, M.A. Environmental factors that enhance the action of the cell penetrating peptide pep-1 A spectroscopic study using lipidic vesicles. Biochim. Biophys. Acta 2005, 1669, 75–86. [Google Scholar]
- Pescina, S.; Govoni, P.; Antopolsky, M.; Murtomäki, L.; Padula, C.; Santi, P.; Nicoli, S. Permeation of Proteins, Oligonucleotide and Dextrans Across Ocular Tissues: Experimental Studies and a Literature Update. J. Pharm. Sci. 2015, 104, 2190–2202. [Google Scholar] [CrossRef]
- Jones, A.; Sayers, E. Cell entry of cell penetrating peptides: Tales of tails wagging dogs. J. Control. Release 2012, 161, 582–591. [Google Scholar] [CrossRef]
- Kauffman, W.; Fuselier, T.; He, J.; Wimley, W.C. Mechanism Matters: A Taxonomy of Cell Penetrating Peptides. Trends Biochem. Sci. 2015, 40, 749–764. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Gong, C.; Ma, Y.; Fan, F.; Luo, M.; Yang, F.; Zhang, Y.-H. Direct cytosolic delivery of cargoes In Vivo by a chimera consisting of D- and L-arginine residues. J. Control. Release 2012, 162, 286–294. [Google Scholar] [CrossRef]
- Yamada, T.; Signorelli, S.; Cannistraro, S.; Beattie, C.W.; Bizzarri, A.R. Chirality Switching within an Anionic Cell-Penetrating Peptide Inhibits Translocation without Affecting Preferential Entry. Mol. Pharm. 2014, 12, 140–149. [Google Scholar] [CrossRef]
- Su, Y.; Doherty, T.; Waring, A.J.; Ruchala, P.; Hong, M. Roles of Arginine and Lysine Residues in the Translocation of a Cell-Penetrating Peptide from13C,31P, and19F Solid-State NMR. Biochemistry 2009, 48, 4587–4595. [Google Scholar] [CrossRef] [Green Version]
- Eiríksdóttir, E.; Konate, K.; Langel, Ü.; Divita, G.; Deshayes, S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochim. et Biophys. Acta (BBA) Biomembr. 2010, 1798, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Juretić, M.; Dukovski, B.J.; Krtalić, I.; Reichl, S.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Lovrić, J.; Pepić, I. HCE-T cell-based permeability model: A well-maintained or a highly variable barrier phenotype? Eur. J. Pharm. Sci. 2017, 104, 23–30. [Google Scholar] [CrossRef]



| Peptide | Sequence a | MW (g/mol) b | Theoretical Charge at pH 7 b |
|---|---|---|---|
| PNT | FAM-RQIKIWFQNRRMKWKK | 2604.61 | +7 |
| PNT-GG | FAM-GG-RQIKIWFQNRRMKWKK | 2718.18 | +7 |
| PNT-GG 1 | FAM-GG-NRRMKWKK | 1617.85 | +5 |
| PNT-GG2 | FAM-GG-FQNRRMKW | 1636.80 | +3 |
| PNT-GG3 | FAM-GG-IWFQNRRM | 1621.79 | +2 |
| PNT-GG4 | FAM-GG-IKIWFQNR | 1575.74 | +2 |
| PNT-GG5 | FAM-GG-RQIKIWFQ | 1589.77 | +2 |
| PNT-R | FAM-GG-KKWKMRRNQFWIKIQR | 2718.18 | +7 |
| PNT-R 1 | FAM-GG-QFWIKIQR | 1589.01 | +2 |
| PNT-R 2 | FAM-GG-RNQFWIKI | 1575.68 | +2 |
| PNT-R 3 | FAM-GG-MRRNQFWI | 1622.75 | +2 |
| PNT-R 4 | FAM-GG-WKMRRNQF | 1636.81 | +3 |
| PNT-R 5 | FAM-GG-KKWKMRRN | 1617.85 | +5 |
| PNT-R-FL | FL-GG-KKWKMRRNQFWIKIQR | 2673.65 | +7 |
| PNT-R-4-FL | FL-GG-WKMRRNQF | 1592.96 | +3 |
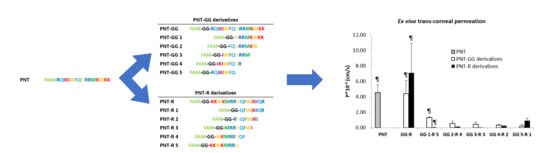
| Peptide | Donor Concentration (µM) | Papp × 10−6 (cm/s) | # Replicates |
|---|---|---|---|
| PNT | 100 a | 4.54 ± 2.01 | 3 |
| PNT-GG analogues | |||
| PNT-GG | 25 a | 4.41 ± 2.93 | 4 |
| PNT-GG | 250 a | n.d. e | 3 |
| PNT-GG 1 | 100 a | 1.33 ± 0.11 | 4 |
| PNT-GG 2 | 100 b | 0.55 ± 0.57 | 3 |
| PNT-GG 3 | 100 b | 0.44 ± 0.50 | 3 |
| PNT-GG 4 | 100 b | 0.34 ± 0.14 | 3 |
| PNT-GG 5 | 100 b | 0.24 ± 0.40 d | 3 |
| 100 c | 0.50 ± 0.70 d | 3 | |
| PNT-R analogues | |||
| PNT-R | 25 a | 7.08 ± 6.66 | 4 |
| PNT-R | 250 a | n.d. e | 3 |
| PNT-R 1 | 250 a | 0.93 ± 0.53 | 4 |
| PNT-R 2 | 250 a | 0.23 ± 0.16 | 3 |
| PNT-R 3 | 250 a | 0.002 ± 0.004 | 3 |
| PNT-R 4 | 250 a | 0.13 ± 0.07 | 4 |
| PNT-R 5 | 250 a | 0.05 ± 0.01 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pescina, S.; Sala, M.; Scala, M.C.; Santi, P.; Padula, C.; Campiglia, P.; Ostacolo, C.; Nicoli, S. Synthesis and Ex Vivo Trans-Corneal Permeation of Penetratin Analogues as Ophthalmic Carriers: Preliminary Results. Pharmaceutics 2020, 12, 728. https://doi.org/10.3390/pharmaceutics12080728
Pescina S, Sala M, Scala MC, Santi P, Padula C, Campiglia P, Ostacolo C, Nicoli S. Synthesis and Ex Vivo Trans-Corneal Permeation of Penetratin Analogues as Ophthalmic Carriers: Preliminary Results. Pharmaceutics. 2020; 12(8):728. https://doi.org/10.3390/pharmaceutics12080728
Chicago/Turabian StylePescina, Silvia, Marina Sala, Maria Carmina Scala, Patrizia Santi, Cristina Padula, Pietro Campiglia, Carmine Ostacolo, and Sara Nicoli. 2020. "Synthesis and Ex Vivo Trans-Corneal Permeation of Penetratin Analogues as Ophthalmic Carriers: Preliminary Results" Pharmaceutics 12, no. 8: 728. https://doi.org/10.3390/pharmaceutics12080728