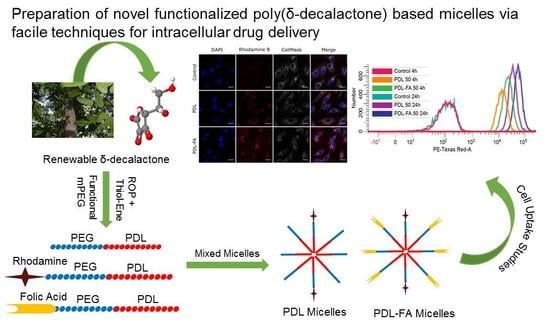
Assessment of Intracellular Delivery Potential of Novel Sustainable Poly(δ-decalactone)-Based Micelles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Instruments
Nuclear Magnetic Resonance (NMR) Spectroscopy
Size Exclusion Chromatography (SEC)
MALDI-TOF Mass Spectrometry (MALDI-TOF Mass)
2.2. Methods
2.2.1. Synthesis of Azide Terminated Poly(ethylene glycol) Methyl Ether (mPEG-N3)
2.2.2. Synthesis of Folate Conjugated Poly(Ethylene Glycol) (FA-PEG-N3)
2.2.3. Synthesis of Rhodamine B Conjugated Poly (ethylene glycol) (RhB-PEG-N3)
2.2.4. Synthesis of Propargyl-PDL
2.2.5. Synthesis of Block Copolymers via Click Chemistry
2.2.6. Preparation and Characterisation of Blank and Drug-Loaded Mixed Micelles
2.2.7. Cell Studies
Cell culture and Maintenance
Cytotoxicity of Blank and DTX Loaded Micelles
Micelles Internalisation and Endocytosis Route Analysis by Flow Cytometry
Cellular Uptake Determination of PDL and PDL-FA Micelles by Confocal Microscopy
2.3. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterisation of Block Copolymers
3.2. Preparation and Characterisation of Block Copolymer Micelles
3.3. Cytotoxicity and Cellular Uptake of Block Copolymer Micelles in MDA-MB-231 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torchilin, V.P. Recent Approaches to Intracellular Delivery of Drugs and DNA and Organelle Targeting. Annu. Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Low, P.S. Folate-Targeted Therapies for Cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate receptors and transporters: Biological role and diagnostic/therapeutic targets in cancer and other diseases. J. Exp. Clin. Cancer Res. 2019, 38, 125. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Cabral, H.; Matsumoto, Y.; Wu, S.; Kano, M.R.; Yamori, T.; Nishiyama, N.; Kataoka, K. Improving Drug Potency and Efficacy by Nanocarrier-Mediated Subcellular Targeting. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Necela, B.M.; Crozier, J.A.; Andorfer, C.A.; Lewis-Tuffin, L.; Kachergus, J.M.; Geiger, X.J.; Kalari, K.R.; Serie, D.J.; Sun, Z.; Aspita, A.M.; et al. Folate Receptor-α (FOLR1) Expression and Function in Triple Negative Tumors. PLoS ONE 2015, 10, e0122209. [Google Scholar] [CrossRef]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.K.; Rosenholm, J.M. Synthetic polymers from renewable feedstocks: An alternative to fossil-based materials in biomedical applications. Ther. Deliv. 2020, 11, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.K.; Gupta, J.; Rosling, A.; Rosenholm, J.M. Renewable poly(δ-decalactone) based block copolymer micelles as drug delivery vehicle: In vitro and in vivo evaluation. Saudi Pharm. J. 2018, 26, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.K.; Kakde, D.; Purdie, L.; Irvine, D.J.; Howdle, S.M.; Mantovani, G.; Alexander, C. New biomaterials from renewable resources—Amphiphilic block copolymers from δ-decalactone. Polym. Chem. 2015, 6, 7196–7210. [Google Scholar] [CrossRef] [Green Version]
- Kakde, D.; Taresco, V.; Bansal, K.K.; Magennis, E.P.; Howdle, S.M.; Mantovani, G.; Irvine, D.J.; Alexander, C. Amphiphilic block copolymers from a renewable ε-decalactone monomer: Prediction and characterization of micellar core effects on drug encapsulation and release. J. Mater. Chem. B 2016, 4, 7119–7129. [Google Scholar] [CrossRef]
- Wik, J.; Bansal, K.K.; Assmuth, T.; Rosling, A.; Rosenholm, J.M. Facile methodology of nanoemulsion preparation using oily polymer for the delivery of poorly soluble drugs. Drug Deliv. Transl. Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wei, X.; Wang, M. Self-Assembled Nanostructures of Red Fluorescent Amphiphilic Block Copolymers as Both Imaging Probes and Drug Carriers. Polymers 2018, 10, 1120. [Google Scholar] [CrossRef] [Green Version]
- Bandelli, D.; Helbing, C.; Weber, C.; Seifert, M.; Muljajew, I.; Jandt, K.D.; Schubert, U.S. Maintaining the Hydrophilic–Hydrophobic Balance of Polyesters with Adjustable Crystallinity for Tailor-Made Nanoparticles. Macromolecules 2018, 51, 5567–5576. [Google Scholar] [CrossRef]
- Bansal, K.K. Novel Amphiphilic Polymers from Renewable Feedstock: Synthesis, Characterisation and Applications. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2015; p. 283. [Google Scholar]
- Opsteen, J.A.; van Hest, J.C.M. Modular synthesis of block copolymers via cycloaddition of terminal azide and alkyne functionalized polymers. Chem. Commun. 2005, 57–59. [Google Scholar] [CrossRef]
- Matini, T.; Francini, N.; Battocchio, A.; Spain, S.G.; Mantovani, G.; Vicent, M.J.; Sanchis, J.; Gallon, E.; Mastrotto, F.; Salmaso, S.; et al. Synthesis and characterization of variable conformation pH responsive block co-polymers for nucleic acid delivery and targeted cell. entry. Polym. Chem. 2014, 5, 1626–1636. [Google Scholar] [CrossRef] [Green Version]
- Azagarsamy, M.A.; Anseth, K.S. Wavelength-Controlled Photocleavage for the Orthogonal and Sequential Release of Multiple Proteins. Angew. Chem.-Int. Ed. 2013, 52, 13803–13807. [Google Scholar] [CrossRef] [Green Version]
- Ladmiral, V.; Mantovani, G.; Clarkson, G.J.; Cauet, S.; Irwin, J.L.; Haddleton, D.M. Synthesis of neoglycopolymers by a combination of “click chemistry” and living radical polymerization. J. Am. Chem. Soc. 2006, 128, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem.-Int. Ed. 2002, 41, 2446–2461. [Google Scholar] [CrossRef]
- Pan, Z.; Yu, L.; Song, N.; Zhou, L.; Li, J.; Ding, M.; Tan, H.; Fu, Q. Synthesis and characterization of biodegradable polyurethanes with folate side chains conjugated to hard segments. Polym. Chem. 2014, 5, 2901–2910. [Google Scholar] [CrossRef]
- Yang, X.; Pilla, S.; Grailer, J.J.; Steeber, D.A.; Gong, S.; Chen, Y.; Chen, G. Tumor-targeting, superparamagnetic polymeric vesicles as highly efficient MRI contrast probes. J. Mater. Chem. 2009, 19, 5812–5817. [Google Scholar] [CrossRef]
- Ding, L.; Hayakawa, T.; Kakimoto, M.-a. Synthesis and characterization of hyperbranched poly(siloxysilane) possessing rhodamine B as terminal group. Polym. J. 2007, 39, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; de Grossi, S.; Riccioli, A.; Amenitsch, H.; Lagana, A. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: From nanostructure to uptake in cancer cells. Nanoscale 2014, 6, 2782–2792. [Google Scholar] [CrossRef]
- Charmainne, C.; Chithrani, D.B. Polyethylene Glycol Density and Length AffectsNanoparticle Uptake by Cancer Cells. J. Nanomed. Res. 2014, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.-Y.; Yan, L.; Zhang, L.; Jin, Y.-M.; Zhao, Q.-H. Folate-modified poly(2-ethyl-2-oxazoline) as hydrophilic corona in polymeric micelles for enhanced intracellular doxorubicin delivery. Int. J. Pharm. 2013, 456, 315–324. [Google Scholar] [CrossRef]
- Pooja, D.; Kulhari, H.; Singh, M.K.; Mukherjee, S.; Rachamalla, S.S.; Sistla, R. Dendrimer–TPGS mixed micelles for enhanced solubility and cellular toxicity of taxanes. Colloids Surf. B Biointerfaces 2014, 121, 461–468. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. In Site-Specific Protein Labeling: Methods and Protocols; Gautier, A., Hinner, M.J., Eds.; Springer: New York, NY, USA, 2015; Volume 1266, pp. 29–53. [Google Scholar]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of Transport Inhibitors on the Cellular Uptake of Carboxylated Polystyrene Nanoparticles in Different Cell Lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef] [Green Version]
- Meier, R.; Henning, T.D.; Boddington, S.; Tavri, S.; Arora, S.; Piontek, G.; Rudelius, M.; Corot, C.; Daldrup-Link, H.E. Breast Cancers: MR Imaging of Folate-Receptor Expression with the Folate-Specific Nanoparticle P1133. Radiology 2010, 255, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Zhang, J.; Sandholm, J.; Lehtimäki, J.; Grönroos, T.; Tuomela, J.; Rosenholm, J.M. Lipid Bilayer-Gated Mesoporous Silica Nanocarriers for Tumor-Targeted Delivery of Zoledronic Acid in Vivo. Mol. Pharm. 2017, 14, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J.; Li, D.; Zhou, S. Polyanhydride micelles with diverse morphologies for shape-regulated cellular internalization and blood circulation. Regen. Biomater. 2017, 4, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francia, V.; Montizaan, D.; Salvati, A. Interactions at the cell membrane and pathways of internalization of nano-sized materials for nanomedicine. Beilstein J. Nanotechnol. 2020, 11, 338–353. [Google Scholar] [CrossRef]
- Sahay, G.; Kim, J.O.; Kabanov, A.V.; Bronich, T.K. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents. Biomaterials 2010, 31, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]









| Sample ID | Mn by 1HNMR (KDa) | Mn by SEC (KDa) | Đ by SEC |
|---|---|---|---|
| mPEG-b-PDL | 12.2 | 21.0 | 1.06 |
| FA-PEG-b-PDL | 12.6 | 23.1 | 1.15 |
| RhB-PEG-b-PDL | 12.7 | 28.4 | 1.25 |
| Sample | Z-Average Size (d/nm) (± SD) | PDI (± SD) | Zeta Potential (mv) (± SD) |
|---|---|---|---|
| PDL micelles | 153 ± 4 | 0.22 ± 0.02 | −2 ± 1 |
| PDL-FA micelles | 147± 3 | 0.14 ± 0.02 | −6 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, K.K.; Özliseli, E.; Saraogi, G.K.; Rosenholm, J.M. Assessment of Intracellular Delivery Potential of Novel Sustainable Poly(δ-decalactone)-Based Micelles. Pharmaceutics 2020, 12, 726. https://doi.org/10.3390/pharmaceutics12080726
Bansal KK, Özliseli E, Saraogi GK, Rosenholm JM. Assessment of Intracellular Delivery Potential of Novel Sustainable Poly(δ-decalactone)-Based Micelles. Pharmaceutics. 2020; 12(8):726. https://doi.org/10.3390/pharmaceutics12080726
Chicago/Turabian StyleBansal, Kuldeep Kumar, Ezgi Özliseli, Gaurav Kumar Saraogi, and Jessica M. Rosenholm. 2020. "Assessment of Intracellular Delivery Potential of Novel Sustainable Poly(δ-decalactone)-Based Micelles" Pharmaceutics 12, no. 8: 726. https://doi.org/10.3390/pharmaceutics12080726