Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers
Abstract
:1. Introduction
2. The Structure of the System
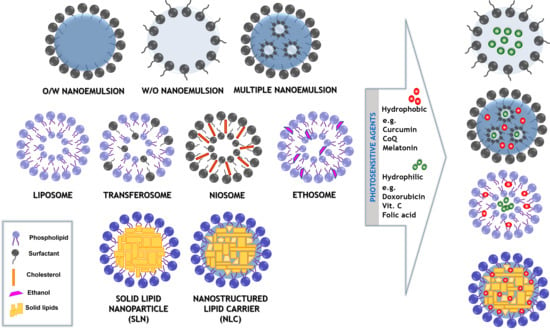
2.1. Nanoemulsions
2.2. Multiple Formulations
2.3. Simple and Modified “Smart” Liposomes
2.4. Nanocarriers Based on Solid Lipids
3. Delivery of Photosensitive Agents
3.1. Advances in Skin Formulations
3.2. Advances in Intravenous Formulations
3.3. Advances in Oral Delivery
4. Concluding Remarks and Future Perspectives
Funding
Conflicts of Interest
References
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siepmann, J.; Faham, A.; Clas, S.D.; Boyd, B.J.; Jannin, V.; Bernkop-Schnürch, A.; Zhao, H.; Lecommandoux, S.; Evans, J.C.; Allen, C.; et al. Lipids and polymers in pharmaceutical technology: Lifelong companions. Int. J. Pharm. 2019, 558, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Wang, P.W.; Alalaiwe, A.; Lin, Z.C.; Fang, J.Y. Use of lipid nanocarriers to improve oral delivery of vitamins. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yan, L. Functional polymer nanocarriers for photodynamic therapy. Pharmaceuticals 2018, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.H.; Inbaraj, B.S. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Ahmad, U.; Akhtar, J.; Badruddeen; Khan, M.M. Engineered nano scale formulation strategies to augment efficiency of nutraceuticals. J. Funct. Foods 2019, 62, 103554. [Google Scholar] [CrossRef]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.S.; Lee, G.Y.; Kim, J.K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2019, 49, 485–517. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.; Mediavilla, M. Recent Patents on Light Based Therapies: Photodynamic Therapy, Photothermal Therapy and Photoimmunotherapy. Recent Pat. Endocr. Metab. Immune Drug Discov. 2014, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. An update on liposomes in drug delivery: A patent review (2014-2018). Expert Opin. Ther. Pat. 2019, 29, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Thakore, S.D.; Patel, M.R. Recent Survey on Patents of Nanoemulsions. Curr. Drug Deliv. 2016, 13, 857–881. [Google Scholar] [CrossRef]
- Date, T.; Nimbalkar, V.; Kamat, J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Control. Release 2018, 271, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Haque, S.; Madheswaran, T.; Zeeshan, F.; Meka, V.S.; Radhakrishnan, A.K.; Kesharwani, P. Lipid based nanocarriers system for topical delivery of photosensitizers. Drug Discov. Today 2017, 22, 1274–1283. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [Green Version]
- Bazylińska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf. B Biointerfaces 2016, 137, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Bazylinska, U.; Saczko, J.; Zielinska, K.; Wilk, K.A. Novel multilayer IR-786-Loaded nanocarriers for intracellular delivering: Characterization, imaging, and internalization in human cancer cell lines. Chem. Lett. 2012, 41, 1354–1356. [Google Scholar] [CrossRef] [Green Version]
- Bazylińska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion structural design in co-encapsulation of hybrid multifunctional agents: Influence of the smart PLGA polymers on the nanosystem-enhanced delivery and electro-photodynamic treatment. Pharmaceutics 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazylińska, U. Rationally designed double emulsion process for co-encapsulation of hybrid cargo in stealth nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 476–482. [Google Scholar] [CrossRef]
- Wawrzyńczyk, D.; Cichy, B.; Zarȩba, J.K.; Bazylińska, U. On the interaction between up-converting NaYF4:Er3+,Yb3+ nanoparticles and Rose Bengal molecules constrained within the double core of multifunctional nanocarriers. J. Mater. Chem. C 2019, 7, 15021–15034. [Google Scholar] [CrossRef]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [Green Version]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Mathew, A.; Marotta, F.; Kumar, D.S. Nanotechnology in Anti-Aging: Nutraceutical Delivery and Related Applications. In Anti-Aging Drugs: From Basic Research to Clinical Practice; Alexander, M., Vaiserman, Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 142–169. [Google Scholar]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.; McClements, D.J. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion & nanoemulsion formation. Food Hydrocoll. 2012, 26, 268–276. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Benoit, J.P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates-A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Lopetinsky, R.J.G.; Masliyah, J.H.; Xu, Z. Solids-Stabilized Emulsions: A Review. In Colloidal Particles at Liquid Interfaces; Cambridge University Press: Cambridge, UK, 2006; ISBN 9780511536670. [Google Scholar]
- Clements, D.J.M.C. Food Emulsions: Principles, Practices and Techniques; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Schulman, J.H.; Montagne, J.B. Formation of Microemulsions By Amino Alkyl Alcohols. Ann. N. Y. Acad. Sci. 1961, 92, 366. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Comparative in vitro evaluation of several colloidal systems, nanoparticles, nanocapsules, and nanoemulsions, as ocular drug carriers. J. Pharm. Sci. 1996, 85, 530–536. [Google Scholar] [CrossRef]
- Elimelech, M.; Gregory, J.; Jia, X.; Williams, R.A. Surface Interaction Potentials. In Particle Deposition & Aggregation; Elsevier: Oxford, UK, 1995; pp. 33–67. [Google Scholar]
- Erramreddy, V.; Ghosh, S. Gelation in nanoemulsion: Structure formation and rheological behavior. In Emulsions; Elsevier Inc.: Saskatoon, SK, Canada, 2016; pp. 257–292. ISBN 9780128043066. [Google Scholar]
- Bhattacharjee, K. Importance of Surface Energy in Nanoemulsion. Nanoemulsions - Prop. Fabr. Appl. 2019, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Nakama, Y. Surfactants. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–244. [Google Scholar]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, J.; Singh, H. Microemulsions: A potential delivery system for bioactives in food. Crit. Rev. Food Sci. Nutr. 2006, 46, 221–237. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade nanoemulsions: Formulation, fabrication, properties, performance, Biological fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro-and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paximada, P.; Mandala, I.; Assadpour, E.; Mehrnia, M.A. 2 – Encapsulation by nanoemulsions. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: San Diego, CA, USA, 2017; pp. 36–73. ISBN 9780128094365. [Google Scholar]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calmon, M.; Bonfim, C.; Monteleoni, L.; Candido, N.; Quintana, S.; Melli, P.; Primo, F.; Adamantino, C.; Antonio, T.; Rahal, P. Effect of curcumin-nanoemulsion associated with photodynamic therapy in HPV-16 E6 positive vulvar carcinoma cell lines. Clin. Cancer Res. 2018, 2018, 4057959. [Google Scholar]
- Matsumoto, S.; Kita, Y.; Yonezawa, D. An attempt at preparing water-in-oil-in-water multiple-phase emulsions. J. Colloid Interface Sci. 1976, 57, 353–361. [Google Scholar] [CrossRef]
- Garti, N.; Bisperink, C. Double emulsions: Progress and applications. Curr. Opin. Colloid Interface Sci. 1998, 3, 657–667. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Ajazuddin; Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheth, T.; Seshadri, S.; Prileszky, T.; Helgeson, M.E. Multiple nanoemulsions. Nat. Rev. Mater. 2020, 5, 214–228. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H.P. Evaluating the Stability of Double Emulsions—A Review of the Measurement Techniques for the Systematic Investigation of Instability Mechanisms. Colloids and Interfaces 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Schmidts, T.; Dobler, D.; Guldan, A.C.; Paulus, N.; Runkel, F. Multiple W/O/W emulsions-Using the required HLB for emulsifier evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 48–54. [Google Scholar] [CrossRef]
- Lokhande, S.S.; Namita, N.; Phalke, N.N.; Raje, V.N.; More, S.S. An Update Review on Recent Advancements in Multiple Emulsion. Int. J. Res. Sci. Innov. 2018, 5, 90–96. [Google Scholar]
- Gharieh, A.; Khoee, S.; Mahdavian, A.R. Emulsion and miniemulsion techniques in preparation of polymer nanoparticles with versatile characteristics. Adv. Colloid Interface Sci. 2019, 269, 152–186. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.A.; Chang, C.B.; Graves, S.M.; Li, Z.; Mason, T.G.; Deming, T.J. Nanoscale double emulsions stabilized by single-component block copolypeptides. Nature 2008, 455, 85–88. [Google Scholar] [CrossRef]
- Ding, S.; Anton, N.; Akram, S.; Er-Rafik, M.; Anton, H.; Klymchenko, A.; Yu, W.; Vandamme, T.F.; Serra, C.A. A new method for the formulation of double nanoemulsions. Soft Matter 2017, 13, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Clegg, P.S.; Tavacoli, J.W.; Wilde, P.J. One-step production of multiple emulsions: Microfluidic, polymer-stabilized and particle-stabilized approaches. Soft Matter 2016, 12, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigward, E.; Mignet, N.; Rat, P.; Dutot, M.; Muhamed, S.; Guigner, J.M.; Scherman, D.; Brossard, D.; Crauste-Manciet, S. Formulation and cytotoxicity evaluation of new self-emulsifying multiple W/O/W nanoemulsions. Int. J. Nanomed. 2013, 8, 611–625. [Google Scholar] [CrossRef] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Olusanya, T.O.B.; Ahmad, R.R.H.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Ortega, V.; Giorgio, S.; De Paula, E. Liposomal formulations in the pharmacological treatment of leishmaniasis: A review. J. Liposome Res. 2017, 27, 234–248. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazù, S.; Kiselev, M.A. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials 2016, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.A.; Bochicchio, S.; Bertoncin, P.; Lamberti, G.; Dalmoro, A. Coating of nanolipid structures by a novel simil-microfluidic technique: Experimental and theoretical approaches. Coatings 2019, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E. Elastic liposomes for topical and transdermal drug delivery. Methods Mol. Biol. 2017, 1522, 107–117. [Google Scholar] [CrossRef]
- Van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katouzian, I.; Faridi Esfanjani, A.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Boukherroub, R.; Szunerits, S. Nanomaterials for transdermal drug delivery: Beyond the state of the art of liposomal structures. J. Mater. Chem. B 2017, 5, 8653–8675. [Google Scholar] [CrossRef]
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef] [Green Version]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Chen, Y.; Li, L.; Lan, P.; He, D.; Song, J.; Zhang, Y. Transdermal Delivery of 5-Aminolevulinic Acid by Nanoethosome Gels for Photodynamic Therapy of Hypertrophic Scars. ACS Appl. Mater. Interfaces 2019, 11, 3704–3714. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Wu, J.; Zhang, D.; Zhang, L.; Song, X.; Hong, H.; He, C.; Mo, X.; Wu, S.; et al. Polyethylenimine and sodium cholate-modified ethosomes complex as multidrug carriers for the treatment of melanoma through transdermal delivery. Nanomedicine 2019, 14, 2395–2408. [Google Scholar] [CrossRef]
- Nasr, S.; Rady, M.; Gomaa, I.; Syrovet, T.; Simmet, T.; Fayad, W.; Abdel-Kader, M. Ethosomes and lipid-coated chitosan nanocarriers for skin delivery of a chlorophyll derivative: A potential treatment of squamous cell carcinoma by photodynamic therapy. Int. J. Pharm. 2019, 568, 118528. [Google Scholar] [CrossRef]
- Lee, E.H.; Lim, S.J.; Lee, M.K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.M.; Müller, R.H.; Bégu, S. Liposomes, lipid nanocapsules and smartCrystals®: A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Shamseddin, A.; Aubert-Pouëssel, A.; Devoisselle, J.M.; Bégu, S. Dermal quercetin lipid nanocapsules: Influence of the formulation on antioxidant activity and cellular protection against hydrogen peroxide. Int. J. Pharm. 2017, 518, 167–176. [Google Scholar] [CrossRef]
- Choudhary, V.; Shivakumar, H.; Ojha, H. Curcumin-loaded liposomes for wound healing: Preparation, optimization, in-vivo skin permeation and bioevaluation. J. Drug Deliv. Sci. Technol. 2019, 49, 683–691. [Google Scholar] [CrossRef]
- Kapoor, M.S.; D’Souza, A.; Aibani, N.; Nair, S.S.; Sandbhor, P.; Kumari, D.; Banerjee, R. Stable Liposome in Cosmetic Platforms for Transdermal Folic acid delivery for fortification and treatment of micronutrient deficiencies. Sci. Rep. 2018, 8, 16122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maione-Silva, L.; De Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campani, V.; Scotti, L.; Silvestri, T.; Biondi, M.; De Rosa, G. Skin permeation and thermodynamic features of curcumin-loaded liposomes. J. Mater. Sci. Mater. Med. 2020, 31, 18. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Shan, X.; Mao, J.; Qiu, L.; Chen, J. Derma roller® microneedles-mediated transdermal delivery of doxorubicin and celecoxib co-loaded liposomes for enhancing the anticancer effect. Mater. Sci. Eng. C 2019, 99, 1448–1458. [Google Scholar] [CrossRef]
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; El Ramly, A. Tretinoin-loaded liposomal formulations: From lab to comparative clinical study in acne patients. Drug Deliv. 2016, 23, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ding, W.; Zhang, Y.; Cheng, S.; Li, F.; Ruan, R.; Wei, P.; Qiu, B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials 2018, 182, 1–12. [Google Scholar] [CrossRef]
- Hatem, S.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Geneidi, A.S.; Elkheshen, S.A. Melatonin vitamin C-based nanovesicles for treatment of androgenic alopecia: Design, characterization and clinical appraisal. Eur. J. Pharm. Sci. 2018, 122, 246–253. [Google Scholar] [CrossRef]
- Bazylińska, U.; Kulbacka, J.; Schmidt, J.; Talmon, Y.; Murgia, S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J. Colloid Interface Sci. 2018, 522, 163–173. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef] [Green Version]
- Kaci, M.; Belhaffef, A.; Meziane, S.; Dostert, G.; Menu, P.; Velot; Desobry, S.; Arab-Tehrany, E. Nanoemulsions and topical creams for the safe and effective delivery of lipophilic antioxidant coenzyme Q10. Colloids Surf. B Biointerfaces 2018, 167, 165–175. [Google Scholar] [CrossRef]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018, 44, 316–328. [Google Scholar] [CrossRef]
- Rajitha, P.; Shammika, P.; Aiswarya, S.; Gopikrishnan, A.; Jayakumar, R.; Sabitha, M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2019, 49, 463–476. [Google Scholar] [CrossRef]
- Sabouri, M.; Samadi, A.; Ahmad Nasrollahi, S.; Farboud, E.S.; Mirrahimi, B.; Hassanzadeh, H.; Nassiri Kashani, M.; Dinarvand, R.; Firooz, A. Tretinoin loaded nanoemulsion for acne vulgaris: Fabrication, physicochemical and clinical efficacy assessments. Skin Pharmacol. Physiol. 2018, 31, 316–323. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; De Holanda e Silva, K.G.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal nanocarriers for enhanced skin delivery of quercetin with functions of anti-tyrosinase and antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashim, I.I.A.; El-Magd, N.F.A.; El-Sheakh, A.R.; Hamed, M.F.; El-Gawad, A.E.G.H.A. Pivotal role of acitretin nanovesicular gel for effective treatment of psoriasis: Ex vivo–in vivo evaluation study. Int. J. Nanomedicine 2018, 13, 1059–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, H.O.; Ghorab, M.M.; Mostafa, D.M.; Ibrahim, E.S. Folic acid loaded lipid nanocarriers with promoted skin antiaging and antioxidant efficacy. J. Drug Deliv. Sci. Technol. 2016, 31, 72–82. [Google Scholar] [CrossRef]
- Ghate, V.M.; Kodoth, A.K.; Raja, S.; Vishalakshi, B.; Lewis, S.A. Development of MART for the Rapid Production of Nanostructured Lipid Carriers Loaded with All-Trans Retinoic Acid for Dermal Delivery. AAPS PharmSciTech 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Silva, L.B.; Kawakami, C.M.; Araújo, M.M.; Del Lama, M.P.F.M.; Naal, R.M.Z.G.; Maria-Engler, S.S.; Gaspar, L.R.; Marcato, P.D. Topical formulation of quercetin encapsulated in natural lipid nanocarriers: Evaluation of biological properties and phototoxic effect. J. Drug Deliv. Sci. Technol. 2019, 53, 101148. [Google Scholar] [CrossRef]
- Dudhipala, N.; Gorre, T. Neuroprotective Effect of Ropinirole Lipid Nanoparticles Enriched Hydrogel for Parkinson’s Disease: In Vitro, Ex Vivo, Pharmacokinetic and Pharmacodynamic Evaluation. Pharmaceutics 2020, 12, 448. [Google Scholar] [CrossRef]
- Ferreira, M.; Barreiros, L.; Segundo, M.A.; Torres, T.; Selores, M.; Costa Lima, S.A.; Reis, S. Topical co-delivery of methotrexate and etanercept using lipid nanoparticles: A targeted approach for psoriasis management. Colloids Surf. B Biointerfaces 2017, 159, 23–29. [Google Scholar] [CrossRef]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.; Gomaa, I.; Afifi, N.; Abdel-Kader, M. Dermal delivery of Fe-chlorophyllin via ultradeformable nanovesicles for photodynamic therapy in melanoma animal model. Int. J. Pharm. 2018, 548, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Pena-Rodríguez, E.; Moreno, M.C.; Blanco-Fernandez, B.; González, J.; Fernández-Campos, F. Epidermal delivery of retinyl palmitate loaded transfersomes: Penetration and biodistribution studies. Pharmaceutics 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.J.; Wang, W.; Xu, X.L.; Jin, F.Y.; Qi, J.; Wang, X.J.; Kang, X.Q.; Zhu, M.L.; Huang, Q.L.; Yu, C.H.; et al. A dual deformable liposomal ointment functionalized with retinoic acid and epidermal growth factor for enhanced burn wound healing therapy. Biomater. Sci. 2019, 7, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-Induced Photosensitivity—An Update: Culprit Drugs, Prevention and Management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef] [PubMed]
- Beiu, C.; Giurcaneanu, C.; Grumezescu, A.M.; Holban, A.M.; Popa, L.G.; Mihai, M.M. Nanosystems for Improved Targeted Therapies in Melanoma. J. Clin. Med. 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Babaie, S.; Del Bakhshayesh, A.R.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A novel nanocarrier for transdermal drug delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.S.; Bae, Y.H. Perspectives on the past, present, and future of cancer nanomedicine. Adv. Drug Deliv. Rev. 2018, 130, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M. Nanocarriers for intravenous injection - The long hard road to the market. Int. J. Pharm. 2013, 457, 50–62. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomed. Pharm. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Amin, M.U.; Ali, M.Y.; Tariq, I.; Pinnapireddy, S.R.; Duse, L.; Goergen, N.; Wölk, C.; Hause, G.; Jedelská, J.; et al. Wavelength dependent photo-cytotoxicity to ovarian carcinoma cells using temoporfin loaded tetraether liposomes as efficient drug delivery system. Eur. J. Pharm. Biopharm. 2020, 150, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Michy, T.; Massias, T.; Bernard, C.; Vanwonterghem, L.; Henry, M.; Guidetti, M.; Royal, G.; Coll, J.L.; Texier, I.; Josserand, V.; et al. Verteporfin-loaded lipid nanoparticles improve ovarian cancer photodynamic therapy in vitro and in vivo. Cancers 2019, 11, 1760. [Google Scholar] [CrossRef] [Green Version]
- Tokarska, K.; Bułka, M.; Bazylińska, U.; Jastrzębska, E.; Chudy, M.; Dybko, A.; Wilk, K.A.; Brzózka, Z. Evaluation of nanoencapsulated verteporfin’s cytotoxicity using a microfluidic system. J. Pharm. Biomed. Anal. 2016, 127, 39–48. [Google Scholar] [CrossRef]
- Park, C.; Yoo, J.; Lee, D.; Jang, S.Y.; Kwon, S.; Koo, H. Chlorin e6-loaded PEG-PCL nanoemulsion for photodynamic therapy and in vivo drug delivery. Int. J. Mol. Sci. 2019, 20, 3958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhao, J.; Hu, H.; Yan, Y.; Hu, X.; Zhou, K.; Xiao, S.; Zhang, Y.; Feng, N. Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6. Int. J. Pharm. 2019, 569, 118595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Meng, X.; Lu, H.; Chang, H.; Dong, H.; Zhang, X. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials 2018, 185, 301–309. [Google Scholar] [CrossRef]
- De Matos, R.P.A.; Calmon, M.F.; Amantino, C.F.; Villa, L.L.; Primo, F.L.; Tedesco, A.C.; Rahal, P. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Cervical Carcinoma Cell Lines. Biomed Res. Int. 2018, 2018, 4057959. [Google Scholar] [CrossRef] [PubMed]
- Oshiro-Junior, J.A.; Sato, M.R.; Boni, F.I.; Santos, K.L.M.; De Oliveira, K.T.; De Freitas, L.M.; Fontana, C.R.; Nicholas, D.; McHale, A.; Callan, J.F.; et al. Phthalocyanine-loaded nanostructured lipid carriers functionalized with folic acid for photodynamic therapy. Mater. Sci. Eng. C 2020, 108, 110462. [Google Scholar] [CrossRef] [PubMed]
- Miretti, M.; Tempesti, T.C.; Prucca, C.G.; Baumgartner, M.T. Zn phthalocyanines loaded into liposomes: Characterization and enhanced performance of photodynamic activity on glioblastoma cells. Bioorganic Med. Chem. 2020, 28, 115355. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, T.S.; Lu, Y.J.; Chen, H.A.; Hsu, H.L.; Jose, G.; Chen, J.P. Dual targeted magnetic photosensitive liposomes for photothermal/photodynamic tumor therapy. J. Magn. Magn. Mater. 2019, 473, 241–252. [Google Scholar] [CrossRef]
- Darwish, W.M.; Bayoumi, N.A.; El-Kolaly, M.T. Laser-responsive liposome for selective tumor targeting of nitazoxanide nanoparticles. Eur. J. Pharm. Sci. 2018, 111, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Im, B.N.; Hwang, H.S.; Na, K. Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials 2018, 183, 139–150. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Di, H.; Luo, L.; Zhu, C.; Yang, J.; Yin, X.; Yin, H.; Gao, J.; Du, Y.; et al. A photosensitive liposome with NIR light triggered doxorubicin release as a combined photodynamic-chemo therapy system. J. Control. Release 2018, 277, 114–125. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Sun, J.; Zhu, S.; Chen, C.; Xie, W.; Zheng, J.; Zhu, Y.; Xiao, L.; Hao, L.; et al. Folate-Targeted and Oxygen/Indocyanine Green-Loaded Lipid Nanoparticles for Dual-Mode Imaging and Photo-sonodynamic/Photothermal Therapy of Ovarian Cancer in Vitro and in Vivo. Mol. Pharm. 2019, 16, 4104–4120. [Google Scholar] [CrossRef]
- Wallenwein, C.M.; Nova, M.V.; Janas, C.; Jablonka, L.; Gao, G.F.; Thurn, M.; Albrecht, V.; Wiehe, A.; Wacker, M.G. A dialysis-based in vitro drug release assay to study dynamics of the drug-protein transfer of temoporfin liposomes. Eur. J. Pharm. Biopharm. 2019, 143, 44–50. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Li, H.; Li, C.; Ding, H.; Zhang, M.; Guo, Y.; Sun, M. Near-infrared light triggered liposomes combining photodynamic and chemotherapy for synergistic breast tumor therapy. Colloids Surf. B Biointerfaces 2019, 173, 564–570. [Google Scholar] [CrossRef]
- Bazylińska, U.; Frąckowiak, R.; Brzózka, Z.; Wilk, K.A. The effect of anionic dicephalic surfactants on fabrication of varied-core nanocarriers for sustained release of porphyrin photosensitizers. J. Photochem. Photobiol. B Biol. 2017, 166, 169–179. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira De Siqueira, L.B.; Da Silva Cardoso, V.; Rodrigues, I.A.; Vazquez-Villa, A.L.; Dos Santos, E.P.; Da Costa Leal Ribeiro Guimarães, B.; Dos Santos Cerqueira Coutinho, C.; Vermelho, A.B.; Junior, E.R. Development and evaluation of zinc phthalocyanine nanoemulsions for use in photodynamic therapy for Leishmania spp. Nanotechnology 2017, 28, 065101. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.T.; De Paula, M.A.; Roatt, B.M.; Garcia, G.M.; Silva, L.S.B.; Reis, A.B.; De Paula, C.S.; Vilela, J.M.C.; Andrade, M.S.; Pound-Lana, G.; et al. Impact of dose and surface features on plasmatic and liver concentrations of biodegradable polymeric nanocapsules. Eur. J. Pharm. Sci. 2017, 105, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Wawrzyńczyk, D. Encapsulation of TOPO stabilized NaYF4:Er3+, Yb3+ nanoparticles in biocompatible nanocarriers: Synthesis, optical properties and colloidal stability. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 556–563. [Google Scholar] [CrossRef]
- Bazylińska, U.; Wawrzyńczyk, D.; Szewczyk, A.; Kulbacka, J. Engineering and biological assessment of double core nanoplatform for co-delivery of hybrid fluorophores to human melanoma. J. Inorg. Biochem. 2020, 111088. [Google Scholar] [CrossRef]
- Bazylińska, U.; Pietkiewicz, J.; Rossowska, J.; Chodaczek, G.; Gamian, A.; Wilk, K.A. Polyelectrolyte Oil-Core Nanocarriers for Localized and Sustained Delivery of Daunorubicin to Colon Carcinoma MC38 Cells: The Case of Polysaccharide Multilayer Film in Relation to PEG-ylated Shell. Macromol. Biosci. 2017, 17, 1600356. [Google Scholar] [CrossRef]
- Kadari, A.; Pooja, D.; Gora, R.H.; Gudem, S.; Kolapalli, V.R.M.; Kulhari, H.; Sistla, R. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur. J. Pharm. Biopharm. 2018, 132, 168–179. [Google Scholar] [CrossRef]
- Kulbacka, J.; Pucek, A.; Kotulska, M.; Dubińska-Magiera, M.; Rossowska, J.; Rols, M.P.; Wilk, K.A. Electroporation and lipid nanoparticles with cyanine IR-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry 2016, 110, 19–31. [Google Scholar] [CrossRef]
- Zheng, G.; Zheng, M.; Yang, B.; Fu, H.; Li, Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: Synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed. Pharm. 2019, 116, 109006. [Google Scholar] [CrossRef]
- Pucek, A.; Niezgoda, N.; Kulbacka, J.; Wawrzeńczyk, C.; Wilk, K.A. Phosphatidylcholine with conjugated linoleic acid in fabrication of novel lipid nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 377–388. [Google Scholar] [CrossRef]
- Kerz, T.; Paret, G.; Herff, H. Routes of drug administration. In Cardiac Arrest, The Science and Practice of Resuscitation Medicine, 2nd ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780511544828. [Google Scholar]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J. Oral drug delivery research in Europe. J. Control. Release 2012, 161, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerns, E.H.; Di, L. Drug-Like Properties: Concepts, Structure Design and Methods, 1st ed.; Academic Press, Elsevier: Burlington, NJ, USA, 2008; ISBN 9780123695208. [Google Scholar]
- Homar, M.; Cegnar, M.; Kotnik, M.; Peternel, L. Toward effective long-term prevention of thromboembolism: Novel oral anticoagulant delivery systems. Semin. Thromb. Hemost. 2010, 36, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.D.; Tamane, P.K.; Khante, S.N.; Pokharkar, V.B. QbD based optimization of curcumin nanoemulsion: DoE and cytotoxicity studies. Indian, J. Pharm. Educ. Res. 2020, 54, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Borrin, T.R.; Georges, E.L.; Moraes, I.C.F.; Pinho, S.C. Curcumin-loaded nanoemulsions produced by the emulsion inversion point (EIP) method: An evaluation of process parameters and physico-chemical stability. J. Food Eng. 2016, 169, 1–9. [Google Scholar] [CrossRef]
- Shukla, M.; Jaiswal, S.; Sharma, A.; Srivastava, P.K.; Arya, A.; Dwivedi, A.K.; Lal, J. A combination of complexation and self-nanoemulsifying drug delivery system for enhancing oral bioavailability and anticancer efficacy of curcumin. Drug Dev. Ind. Pharm. 2017, 43, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hou, Y.; Song, X.; Wang, P.; Li, Y. Cholate-modified polymer-lipid hybrid nanoparticles for oral delivery of quercetin to potentiate the antileukemic effect. Int. J. Nanomed. 2019, 14, 4045–4057. [Google Scholar] [CrossRef] [Green Version]
- Senna, J.P.; Barradas, T.N.; Cardoso, S.; Castiglione, T.C.; Serpe, M.J.; Silva, K.G.d.H.e.; Mansur, C.R.E. Dual alginate-lipid nanocarriers as oral delivery systems for amphotericin B. Colloids Surf. B Biointerfaces 2018, 166, 187–194. [Google Scholar] [CrossRef]
- Ćetković, Z.; Cvijić, S.; Vasiljević, D. Formulation and characterization of novel lipid-based drug delivery systems containing polymethacrylate polymers as solid carriers for sustained release of simvastatin. J. Drug Deliv. Sci. Technol. 2019, 53, 101222. [Google Scholar] [CrossRef]
- Hädrich, G.; Monteiro, S.O.; Rodrigues, M.R.; De Lima, V.R.; Putaux, J.L.; Bidone, J.; Teixeira, H.F.; Muccillo-Baisch, A.L.; Dora, C.L. Lipid-based nanocarrier for quercetin delivery: System characterization and molecular interactions studies. Drug Dev. Ind. Pharm. 2016, 42, 1165–1173. [Google Scholar] [CrossRef]
- Jhan, S.; Pethe, A.M. Double-loaded liposomes encapsulating lycopene β-cyclodextrin complexes: Preparation, optimization, and evaluation. J. Liposome Res. 2020, 30, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zheng, J.; Zhao, L.; Chen, L.L.; Xiong, H.; McClements, D.J. Development of Oral Delivery Systems with Enhanced Antioxidant and Anticancer Activity: Coix Seed Oil and β-Carotene Coloaded Liposomes. J. Agric. Food Chem. 2019, 67, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Panthi, V.K.; Yoon, I.S.; Park, J.W. Preparation, characterization, and in vivo evaluation of an oral multiple nanoemulsive system for co-delivery of pemetrexed and quercetin. Pharmaceutics 2018, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.K.; Kumar, S.; Kaur, H.; Ali, J.; Baboota, S. Attenuation of oxidative damage by coenzyme Q10 loaded nanoemulsion through oral route for the management of Parkinson’s disease. Rejuvenation Res. 2018, 21, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hwang, I.C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Vecchione, R.; Quagliariello, V.; Calabria, D.; Calcagno, V.; De Luca, E.; Iaffaioli, R.V.; Netti, P.A. Curcumin bioavailability from oil in water nano-emulsions: In vitro and in vivo study on the dimensional, compositional and interactional dependence. J. Control. Release 2016, 233, 88–100. [Google Scholar] [CrossRef]
- Pangeni, R.; Kang, S.W.; Oak, M.; Park, E.Y.; Park, J.W. Oral delivery of quercetin in oil-in-water nanoemulsion: In vitro characterization and in vivo anti-obesity efficacy in mice. J. Funct. Foods 2017, 38, 571–581. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Severino, P.; Andreani, T.; Boonme, P.; Santini, A.; Silva, A.M.; Souto, E.B. D-α-tocopherol nanoemulsions: Size properties, rheological behavior, surface tension, osmolarity and cytotoxicity. Saudi Pharm. J. 2017, 25, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Shah, M.R.; Ullah, F.; Ullah, S.; Elhissi, A.M.A.; Nawaz, W.; Ahmad, F.; Sadiq, A.; Ali, I. Sugar-based novel niosomal nanocarrier system for enhanced oral bioavailability of levofloxacin. Drug Deliv. 2016, 23, 3653–3664. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Wu, Y.; Amerigos, D.K.; Chen, Z.; Zhang, M.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. N-acetyl-l-cysteine functionalized nanostructured lipid carrier for improving oral bioavailability of curcumin: Preparation, in vitro and in vivo evaluations. Drug Deliv. 2017, 24, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Xia, Q. Nanostructured lipid carriers incorporated in alginate hydrogel: Enhanced stability and modified behavior in gastrointestinal tract. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 197–206. [Google Scholar] [CrossRef]
- Elmowafy, M.; Ibrahim, H.M.; Ahmed, M.A.; Shalaby, K.; Salama, A.; Hefesha, H. Atorvastatin-loaded nanostructured lipid carriers (NLCs): Strategy to overcome oral delivery drawbacks. Drug Deliv. 2017, 24, 932–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi, H.A.; Allam, A.; Elsabahy, M.; Fetih, G.; El-Badry, M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf. B Biointerfaces 2018, 162, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, K.; Zhou, Y.; Ding, L.; Ullah, A.; Hu, Q.; Sun, M.; Oupický, D. Oral Nanostructured Lipid Carriers Loaded with Near-Infrared Dye for Image-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 25087–25095. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Pucek, A.; Sowa, M.; Matczak-Jon, E.; Wilk, K.A. Engineering of phosphatidylcholine-based solid lipid nanocarriers for flavonoids delivery. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 483–493. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef]
- Dudhipala, N.; Veerabrahma, K. Improved anti-hyperlipidemic activity of Rosuvastatin Calcium via lipid nanoparticles: Pharmacokinetic and pharmacodynamic evaluation. Eur. J. Pharm. Biopharm. 2017, 110, 47–57. [Google Scholar] [CrossRef]
- Kazi, M.; Al-Qarni, H.; Alanazi, F.K. Development of oral solid self-emulsifying lipid formulations of risperidone with improved in vitro dissolution and digestion. Eur. J. Pharm. Biopharm. 2017, 114, 239–249. [Google Scholar] [CrossRef]
- Khattab, A.; Hassanin, L.; Zaki, N. Self-Nanoemulsifying Drug Delivery System of Coenzyme (Q10) with Improved Dissolution, Bioavailability, and Protective Efficiency on Liver Fibrosis. AAPS PharmSciTech 2017, 18, 1657–1672. [Google Scholar] [CrossRef]
- Alwadei, M.; Kazi, M.; Alanazi, F.K. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals–Curcumin and thymoquinone. Saudi Pharm. J. 2019, 27, 866–876. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Abdur Rub, R.; Ahmad, F.J. Enhancement of Quercetin Oral Bioavailability by Self-Nanoemulsifying Drug Delivery System and their Quantification Through Ultra High Performance Liquid Chromatography and Mass Spectrometry in Cerebral Ischemia. Drug Res. (Stuttg). 2017, 67, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Kushwah, V.; Thanki, K.; Jain, S. Triple antioxidant SNEDDS formulation with enhanced oral bioavailability: Implication of chemoprevention of breast cancer. Nanomed. Nanotechnol., Biol. Med. 2016, 12, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khursheed, R.; Kumar, R.; Awasthi, A.; Sharma, N.; Khurana, S.; Kapoor, B.; Khurana, N.; Singh, S.K.; Gowthamarajan, K.; et al. Self-nanoemulsifying drug delivery system of fisetin: Formulation, optimization, characterization and cytotoxicity assessment. J. Drug Deliv. Sci. Technol. 2019, 54, 101252. [Google Scholar] [CrossRef]
- Qin, L.; Niu, Y.; Wang, Y.; Chen, X. Combination of Phospholipid Complex and Submicron Emulsion Techniques for Improving Oral Bioavailability and Therapeutic Efficacy of Water-Insoluble Drug. Mol. Pharm. 2018, 15, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Condat, M.; Babinot, J.; Tomane, S.; Malval, J.P.; Kang, I.K.; Spillebout, F.; Mazeran, P.E.; Lalevée, J.; Andalloussi, S.A.; Versace, D.L. Development of photoactivable glycerol-based coatings containing quercetin for antibacterial applications. RSC Adv. 2016, 6, 18235–18245. [Google Scholar] [CrossRef]
- Orozco, D.; Skamarack, J.; Reins, K.; Titlow, B.; Lunetta, S.; Li, F.; Roman, M. Determination of ubidecarenone (coenzyme Q10, ubiquinol-10) in raw materials and dietary supplements by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation. J. AOAC Int. 2007, 90, 1227–1236. [Google Scholar] [CrossRef] [Green Version]
- Prasad, T.; Kalaiselvan, T.; Surabhi, S.; Vivek, D.; Ranvirkumar, S.; Gyanendra Nath, S. Atorvastatin Induced Vasculitis. Indian J. Pharm. Pract. 2014, 7, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Mikulich, A.V.; Tretyakova, A.I.; Knukshto, V.N.; Plavskaya, L.G.; Leusenka, I.A.; Ananich, T.S.; Plavskii, V.Y.; Ulaschik, V.S. Potential of Antifungal Drugs as Photosensitizers. KnE Energy 2018, 3, 223. [Google Scholar] [CrossRef]




| Formulation | Surfactant/Co-Surfactant (Non-Lipid Origin) | Oil/Lipid | Photosensitive Cargo | DH [nm] | EE [%] | Cargo Detection Method | Application | Ref |
|---|---|---|---|---|---|---|---|---|
| Ethosomes | - | Phosphatidylcholine | 5-Aminolevulinic acid | 81 | 53 | FM | Hypertrophic scars | [91] |
| Ethosomes | Polyethyleneimine, sodium cholate | Phosphatidylcholine, cholesterol | Curcumin, doxorubicin | 50–350 | - | FM | Melanoma | [92] |
| Ethosomes | Cremophor A25 | Phosphatidylcholine | Ferrous chlorophyllin | 383 | 78 | UV-Vis spectroscopy, CLSM | Squamous cell carcinoma of the skin, PDT | [93] |
| Chitosan-coated liposomes | - | Phosphatidylcholine | 201 | 68 | ||||
| Chitosan-coated liposomes | - | DMPC, cholesterol | Indocyanine green | 231–1983 | - | UV-Vis spectroscopy, FM | Melanoma, PDT | [94] |
| Lipid nanocapsules | Solutol HS 15, Cremophor EL, Labrafac Lipophile WL 1349 | Lipoid S75-3 | Quercetin | 27–54 | 90–96 | HPLC with ultraviolet detector | Anti-inflammatory activity, psoriasis, atopic dermatitis | [95,96] |
| Liposomes | Cremophor EL | DPPC | Quercetin | 179 | 68 | HPLC with ultraviolet detector | UV-protection | |
| Liposomes | - | Phosphatidylcholine, cholesterol | Curcumin | 189–395 | 76–84 | UV–Vis spectroscopy, FM | Wound healing | [97] |
| Liposomes | Oleic acid | Phosphatidylcholine | Folic acid | 120–280 | 6–70 | CLSM | Treatment of micronutrient deficiencies | [98] |
| Liposomes | - | Phosphatidylcholine, cholesterol, DOTAP, DSPG | Ascorbic acid | 161–190 | 17–58 | HPLC with diode array detector, FM | Anti-aging, UV-A protection | [99] |
| Liposomes | - | DPPC | Curcumin | 104–133 | 31–43 | HPLC with ultraviolet detector | Anti-inflammatory activity, psoriasis, melanoma | [100] |
| Liposomes | - | HSPC, cholesterol | Doxorubicin, celecoxib | 121–142 | 98–99 | UV-Vis spectroscopy | Melanoma | [101] |
| Liposomes | Dicetyl phosphate | Phosphatidylcholine, cholesterol | Tretinoin | 318–485 | 38–73 | UV-Vis spectroscopy | Acne | [102] |
| Peptide functionalized liposomes | Sodium cholate | Phosphatidylcholine, cholesterol | Vemurafenib | 73–105 | 98 | UV-Vis spectroscopy | Melanoma | [103] |
| Aspasomes | Dicetyl phosphate | Cholesterol, ascorbyl palmitate | Melatonin | 287–950 | 52–91 | UV-Vis spectroscopy | Androgenic alopecia | [104] |
| Cubosomes | Propylene glycol | Glycerol monooleate, Lipoid S75 | Chlorin e6 | 138 | 97 | UV-Vis spectroscopy, CLSM | Melanoma, PDT | [105] |
| TPP-Mn | 146 | 91 | ||||||
| Nanoemulsions | Tween 80/ PEG 400 | Clove oil | Curcumin | 93 | - | DSC | Wound healing, anti-inflammatory activity | [106] |
| Nanoemulsions | Rapeseed lecithin | Rapeseed oil | Coenzyme Q10 | 123–158 | 93 | UV-Vis spectroscopy, epi-FM | Anti-wrinkle and anti-inflammatory activities, UV-protection, skin disease (e.g., facial vitiligo) | [107] |
| Nanoemulsions | Tween 80/ Transcutol HP | Isopropyl myristate | Coenzyme Q10 | 11 | - | HPLC | Anti-wrinkle activity | [108] |
| Nanoemulsions | Tween 80/ ethanol | Chaulmoogra oil | Methotrexate | 34 | 88 | UV-Vis spectroscopy, FM | Psoriasis | [109] |
| Nanoemulsions | Tween 80 | Crodamol GTCC | Tretinoin | 116 | 99 | UV-Vis spectroscopy | Acne | [110] |
| Nanoemulsions | Pluronic F68 | Clove oil | 8-Methoxypsoralen | 91 | - | HPLC with ultraviolet detector | Psoriasis, vitiligo | [111] |
| Cremophor RH40 | Sweet fennel oil | 68 | - | |||||
| Niosomes | Span 60, Cremophor RH40 | - | Quercetin | 97 | 87 | HPLC | Hyperpigmentation | [112] |
| Niosomes | Span 60 | Cholesterol | Acitretin | 369 | 90 | UV-Vis spectroscopy | Psoriasis | [113] |
| NLCs | Tween 80, Labrafil M 2130 CS | Capryol 90, Captex 355, Geleol, Apifil | Folic acid | 50–94 | 95–98 | UV-Vis spectroscopy | Anti-aging | [114] |
| NLCs | Tween 80, Span 60 | Avacado oil, grape seed oil/stearic acid, cetyl alcohol, glyceryl monostearate | All-trans retinoic acid | 67 | >98 | HPLC with ultraviolet detector | Acne, photo-aging, eczema, psoriasis | [115] |
| NLCs | Pluronic F68 | Calendula oil, Illipe butter | Quercetin | 130 | 97 | UV-Vis spectroscopy | Anti-aging, UV-B protection | [116] |
| SLNs | Poloxamer 188 | Dynasan 114, soy lecithin | Ropinirole | 211 | 77 | HPLC | Parkinson’s disease | [117] |
| NLCs | Dynasan 114, Capryol 90, soy lecithin | 193 | 84 | |||||
| Antibody functionalized SLNs | Tween 80 | Cetyl palmitate | Methotrexate | 292–356 | 85–88 | HPLC with photodiode array detector | Psoriasis | [118] |
| SLNs | Tween 80 | Precirol ATO 5 | Curcumin | 51 | 93 | HPLC | Hyperpigmentation irritant contact dermatitis | [119] |
| Transferosomes | Tween 20, Tween 80, Span 20, Cremophor A25 | Phosphatidylcholine | Ferrous chlorophyllin | 284–651 | 37–56 | UV-Vis spectroscopy | Melanoma | [120] |
| Transferosomes | Tween 80 | Phosphatidylcholine | Retinyl palmitate | 300 | 100 | HPLC with photodiode array detector, FM | Anti-aging, hyperpigmentation | [121] |
| Transferosomes | Tween 80 | Phosphatidylcholine | All-trans retinoic acid | 48–87 | 99 | HPLC, CLSM | Wound healing, treatment of deep partial-thickness burns | [122] |
| Formulation | Surfactant/Co-Surfactant (Non-Lipid Origin) | Oil/Lipid | Photosensitive Cargo | DH [nm] | EE [%] | Cargo Detection Method | Application | Ref |
|---|---|---|---|---|---|---|---|---|
| Liposomes | - | DPPC/cholesterol, DPPC/DPPE-mPEG5000 or DPPC/TEL | Temoporfin | 106–129 | 78–90 | UV-Vis spectroscopy, CLSM | PDT | [132] |
| Magnetic photosensitive liposomes | DDAB | DSPC, cholesterol | Indocyanine green | 222 | 12 | UV-Vis spectroscopy | PTT/PDT | [141] |
| Liposomes | Triton X-100 | Phosphatidylcholine | Zinc-phthalocyanine star polymer, nitazoxanide | 87 | - | UV-Vis spectroscopy | Bioimaging, PDT | [142] |
| Liposomes | - | DPPC, cholesterol, DOPE, CHEMS | Gemcitabine, pheophorbide A | 102 | 37 | HPLC, CLSM | PDT, chemotherapy | [143] |
| Antibody functionalized liposomes | - | Phosphatidylcholine, cholesterol, DSPE-PEG2000 | Doxorubicin, modified indocyanine green | 128 | >90 | UV-Vis spectroscopy, FS | PDT, chemotherapy | [144] |
| Liposomes | - | DPPC, DPPG, DSPE-PEG2000-folate, cholesterol | Indocyanine green | 301 | 96 | UV-Vis spectroscopy, FS, CLSM | Diagnostics, PTT, sono-PDT photo-sonodynamic combined therapy | [145] |
| Liposomes | - | DPPC, cholesterol | ZnPc, TAZnPc | 102–190 | 74–75 | UV-Vis spectroscopy, FS | PDT | [140] |
| Liposomes | - | Lipoid S100 | Temoporfin | 141 | 82 | HPLC with fluorescence detector | PDT | [146] |
| Liposomes | - | Phosphatidylcholine cholesterol | IR-780 | 130 | 67 | UV-Vis spectroscopy, CLSM | PDT | [147] |
| Liposomes | - | Lecithin, DSPE-PEG2000, cholesterol | Chlorin e6 | 162 | 82 | UV-Vis spectroscopy, FS, CLSM | Diagnostics, PDT | [137] |
| Nanoemulsions | Anionic dicephalic surfactants CnH2n+1-N-(CH2COONa)2 n = 10, 12 or 14 | Isopropyl myristate or palm oil | Verteporfin or meso-tetraphenylporphyrin | 129–170 | 85–98 | UV-Vis spectroscopy | PDT | [148] |
| Nanoemulsions | Poloxamer 188 | Lipoid S100 | Curcumin | 199 | - | UV-Vis spectroscopy, FM, FS | PDT | [138] |
| Nanoemulsions | Pluronic F127 | Clove oil | ZnPc | 30–202 | - | UV-Vis spectroscopy | PDT | [149] |
| Nanoemulsions | Pluronic F68 | Miglyol 812 N, Epikuron TM | AlClPc | 133 | >99 | HPLC | Diagnosis, PDT | [150] |
| Nanoemulsions | PEG-b-PCL | Soybean oil | Chlorin e6 | 220 | - | FM, FS | PDT | [135] |
| Multiple nanoemulsions (polymeric double-core NCs) | Di-C12DMAB and Cremophor A25, Cremophor RH 40 or Poloxamer 407 | PEG-PLGA, PEG-PCL, PEG-PDLLA in DCM | DNA, thiazole orange | 143–184 | 72–95 | UV-Vis spectroscopy | Gene therapy, bioimaging | [22] |
| Multiple nanoemulsions (polymeric double-core NCs) | Span 80, Cremophor A25 | PLGA in DCM | NaYF4:Er3+,Yb3+NPs | 134–265 | - | NIR spectroscopy and spectrofluorimetry | NIR-induced imaging | [151] |
| Multiple nanoemulsions (”smart” double-core polymeric NCs) | Di-C12DMAB, Cremophor A25 | PLGA, PEG-PLGA, FA-PLGA in DCM | Verteporfin, cisplatin | 187–200 | 88–97 | UV-Vis spectroscopy, CLSM, FACS | Combined chemo- and EP-PDT | [21] |
| Multiple nanoemulsions (”smart” double-core polymeric NCs) | Span 80, Rosulfan A | PLGA in DCM | NaYF4:Er3+,Yb3+NPs, Rose Bengal | 127–154 | - | NIR spectroscopy and spectrofluorimetry | Theranostics, NIR-induced imaging and PDT | [23] |
| Multiple nanoemulsions (double-core polymeric NCs) | Span 80, Cremophor A25 | PLGA in DCM | NaYF4:Er3+,Yb3+NPs+, Rose Bengal | 150–158 | - | NIR spectroscopy and spectrofluorimetry CLSM | Theranostics, NIR-induced bioimaging and PDT | [152] |
| Nanoemulsion-based multilayer NCs | Quaternary ammonium gemini surfactants: d(DDA)PBr and d(DDA)BBr | Isopropyl myristate, oleic acid | IR-780 | 101–119 | >90 | UV-Vis spectroscopy, FM, CLSM | NIR-induced bioimaging | [19] |
| Nanoemulsion-based multilayer NCs | Cationic surfactant C12(TAPAMS)2 | Oleic acid | Daunorubicin | 103–120 | 86–96 | UV-Vis spectroscopy, CLSM | Chemotherapy | [153] |
| Nanoemulsion-based multilayer NCs | Cationic surfactant C12(TAPAMS)2 | Oleic acid | Verteporfin | 118 | 92 | UV-Vis spectroscopy, FM, CLSM | Diagnostics, PDT | [134] |
| SLNs | Tween 80 | Glyceryl monostearate, stearic acid, soya lecithin | Docetaxel | 79–111 | 87–90 | HPLC, FM | Chemotherapy | [154] |
| SLNs | Tween 80 | Cetyl palmitate, Phospholipon 90G | IR-780 | 134–237 | 22–63 | UV-Vis spectroscopy, CLSM | EP-PDT | [155] |
| SLNs | Myrj 52 | Glycerol monostearate, lecithin | Doxorubicin | 81–96 | 89–90 | HPLC, FM | Chemotherapy | [156] |
| NLCs | Myrj S40 | Suppocire NB, soybean oil, Lipoid S75 | Verteporfin | 50 | >95 | HPLC, FM, CLSM | PDT | [133] |
| NLCs | Pluronic F127, Polyoxyethylene 40 stearate, ethoxylated hydrogenated castor oil | Capric/caprylic acid triglycerides | Zinc phthalocyanine | 165 | 63 | FS | PDT | [139] |
| NLCs | Tween 80 | Cetyl palmitate, Miglyol 812 N, (CLA)PC | IR-780 | 159–228 | - | CLSM | Bioimaging | [157] |
| NLCs | Cremophor RH40, DSPE-PEG2000 | Precirol ATO5, and Maisine 35-1 | Chlorin e6 and paclitaxel | 121 | 93–94 | HPLC, FM, CLSM | PDT, chemotherapy | [136] |
| Formulation | Surfactant /Co-Surfactant (Non- Lipid Origin) | Oil/Lipid | Photosensitive Cargo | DH [nm] | EE [%] | Cargo Detection Method | Application | Ref |
|---|---|---|---|---|---|---|---|---|
| Cholate-modified polymer-lipid hybrid nanoparticles | - | PLGA, Lipoid S100 | Quercetin | 110 | 96 | HPLC, CLSM | Antileukemic activity | [166] |
| Dual alginate-lipid nanocarriers | Tween 20, Span 80, Kolliphor P188 | Glyceryl monostearate, Miglyol 812N | Amphotericin B | 83–120 | 78–81 | HPLC with photodiode array detector | Antimicrobial/ antifungal-PDT | [167] |
| Gel-like lipid-based drug delivery systems | PEG 400 caprylic/capric glycerides/PEG-15 hydroxystearate | PEG 300 oleic glycerides, propylene glycol monolaurate and monocaprylate | Simvastatin | 13–23 | - | UV-Vis spectroscopy | Antihyperlipidemic activity | [168] |
| Lipid-based nanocarriers | PEG 660-stearate | Castor oil, Phospholipon 80 | Quercetin | 20 | - | HPLC with ultraviolet detector, NMR spectroscopy | Antioxidant and anti-inflammatory activity | [169] |
| Double-loaded liposomes | - | Phosphatidylcholine, cholesterol | Lycopene | 143–652 | 58–84 | UV−Vis spectroscopy, DSC, FTIR | Cardioprotective activity | [170] |
| Liposomes | - | Phosphatidylcholine, cholesterol | β-carotene | 129 | 86 | UV−Vis spectroscopy | Functional foods and supplements | [171] |
| Multiple nanoemulsions | Labrasol/Tween 80, Cremophor EL, PEG 400 | Labrafil M 1944 CS | Quercetin | 15 | - | CLSM | Anticancer therapy | [172] |
| Nanoemulsions | Tween 20, Tween 80, Solutol HS 15, Unitop FFT 40/Transcutol P,ethanol, PEG 400 | Vitamin E, Capmul MCM, Labrafac Lipophile WL 1349, Captex, Capryol 90 | Coenzyme Q10 | 20–31 | - | HPLC with ultraviolet detector | Parkinson’s disease treatment | [173] |
| Nanoemulsions | Tween 80 | Soybean oil | Curcumin | 198–272 | - | UV–Vis spectroscopy | Food formulations | [164] |
| Nanoemulsions | Tween 80 | MCT oil, lecithin | Curcumin | 114 | 95 | UV–Vis spectroscopy | Functional food and beverage system | [174] |
| Nanoemulsions | - | Soybean oil, Lipoid E80 | Curcumin | 110 | - | CLSM, STED | Anti-inflammatory and anticancer activities | [175] |
| Nanoemulsions | Tween 80/TPGS | Kollisolv MCT 70 | Curcumin | 17 | 98 | UV–Vis spectroscopy | Anticancer activity against pituitary and colon cell lines | [163] |
| Nanoemulsions | Tween 20, Tween 80, Span 80, Labrasol/ Cremophor EL, PEG 400, Transcutol HP, Plurol Oleique CC | Castor oil, oleic acid, Capryol 90, Labrafil M 1944 | Quercetin | 19–126 | - | HPLC with ultraviolet detector | Obesity treatment | [176] |
| Nanoemulsions | Tween 80 | Lipoid S75, Miglyol 812 N | D-α-tocopherol | 65–90 | - | - | Antioxidant activity | [177] |
| Niosomes | Non-ionic surfactant BRD-BG | Cholesterol | Levofloxacin | 190 | 68 | HPLC, FTIR | Antibiotic therapy | [178] |
| NLCs | - | Phosphatidylcholine, cholesterol oleate, | Curcumin | 141 | 92 | HPLC, CLSM | Antioxidant and anticancer activity | [179] |
| NLCs | Tween 80 | Glycerol monostearate, octyl and decyl glycerate | Quercetin | 86 | 98 | UV–Vis spectroscopy | Anti-inflammatory and anticancer activities | [180] |
| NLCs | Pluronic F68, Tween 80 | Gelucire 43/01, Capryol PGMC, GMS, lecithin | Atorvastatin | 163–866 | 76–97 | UV-Vis spectroscopy | Antihyperlipidemic activity | [181] |
| NLCs | Pluronic F68 | Stearic acid, oleic acid, lecithin | Simvastatin | 169 | 40–76 | UV-Vis spectroscopy, FTIR | Antihyperlipidemic activity | [182] |
| NLCs | - | Labrafac CC, trilaurin, soy lecithin | IR-780 | 170 | - | UV−Vis spectroscopy | Photothermal anticancer therapies | [183] |
| SLNs | - | Phosphatidylcholine, Polawax NF | Baicalein, myricetin, flavonoids cocrystals | 45–104 | 51–92 | UV–Vis spectroscopy, FTIR, XRPD | Antioxidant, antitumor and anti-inflammatory activities | [184] |
| SLNs | TPGS | Cholesterol, palmitic acid | Curcumin | 139 | 93 | HPLC with ultraviolet detector, FTIR | Brain gliomas and Alzheimer’s disease treatments | [185] |
| SLNs | Poloxamer 188 | Dynasan 112, egg lecithin | Rosuvastatin calcium | 67 | 94 | HPLC | Antihyperlipidemic activity | [186] |
| SELFs | HCO-30, TO-106V, Transcutol P | Coconut oil, CremerCOOR, MCT 70/30, Capmul MCM, Imwitor 988, 308 | Risperidone | 16–111 | - | FTIR | Antipsychotic activity | [187] |
| SNEDDS | Cremophor EL, Labrasol, Tween 80/Transcutol | Isopropyl myristate | Coenzyme Q10 | 11–13 | - | HPLC | Protection against liver fibrosis and cirrhosis | [188] |
| SNEDDS | Tween 80, PEG 400 | Castor oil | Curcumin | 83 | - | HPLC, UV-Vis spectroscopy | Anticancer activity against metastatic breast cancer cells | [165] |
| SNEDDS | Transcutol P, Cremophor EL, Cremophor RH40, hydrogenated castor oil | Black seed oil, Imwitor 988 | Curcumin | 18–25 | - | FTIR, ultra-HPLC | Anti-inflammatory and anticancer treatments | [189] |
| SNEDDS | PEG 400, Tween 20, Tween 60, Tween 80, Labrasol, Cremophor EL | Sesame oil, oleic acid, isopropyl myristate, olive oil, ethyl oleate | Quercetin | 27–249 | - | UV–Vis spectroscopy, ultra-HPLC | Brain tumor | [190] |
| SNEDDS | Cremophor RH40, Labrafil1944 CS | Capmul MCM EP | Quercetin, resveratrol | 62–214 | - | HPLC, CLSM | Antioxidant therapies | [191] |
| SNEDDS | Lauroglycol FCC, Tween 80/Transcutol P | Castor oil | Fisetin | 154–157 | 58–105 | HPLC with photodiode array detector | The cancer, Neurodegenerative disorders treatments | [192] |
| Nanoemulsions | Pluronic F68, Tween 80, sodium oleate/glycerin | Soybean oil, ovolecithin | Atorvastatin | 123–151 | 98 | HPLC, FTIR | Antihyperlipidemic activity | [193] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. https://doi.org/10.3390/pharmaceutics12060587
Pucek A, Tokarek B, Waglewska E, Bazylińska U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics. 2020; 12(6):587. https://doi.org/10.3390/pharmaceutics12060587
Chicago/Turabian StylePucek, Agata, Beata Tokarek, Ewelina Waglewska, and Urszula Bazylińska. 2020. "Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers" Pharmaceutics 12, no. 6: 587. https://doi.org/10.3390/pharmaceutics12060587