In Vitro Skin Retention of Crisaborole after Topical Application
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
2.2.1. Thermal Analysis
2.2.2. Solubility Determination
2.2.3. HPLC Analysis
2.2.4. Determination of Octanol/Water Partition Coefficient
2.2.5. Ointment Preparation and Characterization
2.2.6. Tissue Preparation
2.2.7. Skin Permeation and Retention
2.2.8. Data Analysis
3. Results and Discussion
3.1. Crisaborole Characterization
3.1.1. Thermal Analysis
3.1.2. Partition Coefficient
3.1.3. Solubility
3.2. Crisaborole Skin Permeation and Retention from Ointment
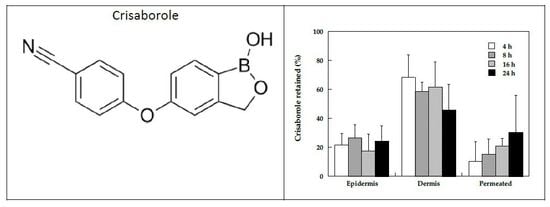
3.2.1. Time Course of Crisaborole Skin Penetration and Retention
3.2.2. Stratum Corneum Distribution
3.2.3. Crisaborole Distribution in the Skin Compartments
3.2.4. The Role of Stratum Corneum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef]
- Kapur, S.; Watson, W.; Carr, S. Atopic dermatitis. Allergy Asthma Clin. Immunol. 2018, 14, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.; Simpson, E.L. The Socioeconomics of Atopic Dermatitis. Ann. Allergy Asthma Immunol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Tham, E.H.; Leung, D.Y.M. Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. Allergy Asthma Immunol. Res. 2019, 11, 4. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 1027, pp. 21–37. [Google Scholar]
- Deleanu, D.; Nedelea, I. Biological therapies for atopic dermatitis: An update (Review). Exp. Ther. Med. 2018, 17, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paller, A.S.; Kabashima, K.; Bieber, T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immunol. 2017, 140, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Xu, J.; Luo, D. Efficacy and tolerance of tacrolimus and pimecrolimus for atopic dermatitis: A meta-analysis. J. Biomed. Res. 2011, 25, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Paller, A.S.; Boguniewicz, M.; Eichenfield, L.F.; Feldman, S.R.; Silverberg, J.I.; Chamlin, S.L.; Zane, L.T. Crisaborole Ointment Improves Quality of Life of Patients with Mild to Moderate Atopic Dermatitis and Their Families. Dermatol. Ther. (Heidelb.) 2018, 8, 605–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, A.B.; Gor, A.; Bui, M.R. Topical Crisaborole—A Potential Treatment for Recalcitrant Palmoplantar Psoriasis. JAMA Dermatol. 2018, 154, 1096. [Google Scholar] [CrossRef] [PubMed]
- Makins, C.; Sanghera, R.; Grewal, P.S. Off-Label Therapeutic Potential of Crisaborole. J. Cutan. Med. Surg. 2020, 1203475420909794. [Google Scholar] [CrossRef] [PubMed]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig Ear Skin ex Vivo as a Model for in Vivo Dermatopharmacokinetic Studies in Man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.P.; Scott, R.C. Pig Ear Skin as an In-vitro Model for Human Skin Permeability. J. Pharm. Pharmacol. 1992, 44, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; Padula, C. Development and validation of a simple method for the extraction and quantification of crisaborole in skin layers. Biomed. Chromatogr. 2019. [Google Scholar] [CrossRef]
- WO2017093857A1 - Topical Pharmaceutical Formulations for Treating Inflammatory-Related Conditions - Google Patents. Available online: https://patents.google.com/patent/WO2017093857A1/en (accessed on 17 April 2020).
- Simonsen, L.; Fullerton, A. Development of an in vitro Skin Permeation Model Simulating Atopic Dermatitis Skin for the Evaluation of Dermatological Products. Skin Pharmacol. Physiol. 2007, 20, 230–236. [Google Scholar] [CrossRef]
- Padula, C.; Campana, N.; Santi, P. Simultaneous determination of benzophenone-3, retinol and retinyl acetate in pig ear skin layers by high-performance liquid chromatography. Biomed. Chromatogr. 2008, 22, 1060–1065. [Google Scholar] [CrossRef]
- Padula, C.; Colombo, G.; Nicoli, S.; Catellani, P.L.; Massimo, G.; Santi, P. Bioadhesive film for the transdermal delivery of lidocaine: In vitro and in vivo behavior. J. Control. Release 2003, 88, 277–285. [Google Scholar] [CrossRef]
- Campillo-Alvarado, G.; Didden, T.D.; Oburn, S.M.; Swenson, D.C.; Macgillivray, L.R. Exploration of Solid Forms of Crisaborole: Crystal Engineering Identifies Polymorphism in Commercial Sources and Facilitates Cocrystal Formation. Cryst. Growth Des. 2018, 18, 4416–4419. [Google Scholar] [CrossRef]
- Ceric, H.; Malenica, M.; Ratkaj, M.; Landeka, I.; Jegorov, A. Solid State Forms of Crisaborole. U.S. Patent EP 3484895 A1, 22 May 2019. [Google Scholar]
- Mitragotri, S.; Anissimov, Y.G.; Bunge, A.L.; Frasch, H.F.; Guy, R.H.; Hadgraft, J.; Kasting, G.B.; Lane, M.E.; Roberts, M.S. Mathematical models of skin permeability: An overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849372483. [Google Scholar]
- Sloan, K.B.; Koch, S.A.M.; Siver, K.G.; Flowers, F.P. Use of solubility parameters of drug and vehicle to predict flux through skin. J. Invest. Dermatol. 1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lademann, J.; Otberg, N.; Jacobi, U.; Hoffman, R.M.; Blume-Peytavi, U. Follicular penetration and targeting. J. Investig. Dermatol. Symp. Proc. 2005, 10, 301–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Solvent | Solubility Parameter (MPa1/2) | Crisaborole Solubility (mg/mL) |
|---|---|---|
| Octanol | 20.87 [25] | 75.80 ± 1.70 |
| Ethanol | 26.52 [25] | 40.6 ± 11.3 |
| Propylene Glycol | 30.22 [25] | 12.20 ± 2.55 |
| Oleic acid | 17.39 [25] | 3.44 ± 0.33 |
| Isopropyl myristate | 17.38 [26] | 3.43 ± 1.68 |
| Water | 47.8 [25] | 0.11 ± 0.01 |
| 2% Ointment | 2% ACN Solution [17] | |||
|---|---|---|---|---|
| Intact Skin | Damaged Skin | Intact Skin | ||
| Contact Time (h) | Epidermis/Dermis Retention | Stratum Corneum Distribution | Epidermis/Dermis Retention | Epidermis/Dermis Retention |
| 4 | X (4) | X (4) | X (4) | |
| 8 | X (8) | |||
| 16 | X (4) | X (4) | X (6) | |
| 24 | X (7) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantini, A.; Demurtas, A.; Nicoli, S.; Padula, C.; Pescina, S.; Santi, P. In Vitro Skin Retention of Crisaborole after Topical Application. Pharmaceutics 2020, 12, 491. https://doi.org/10.3390/pharmaceutics12060491
Fantini A, Demurtas A, Nicoli S, Padula C, Pescina S, Santi P. In Vitro Skin Retention of Crisaborole after Topical Application. Pharmaceutics. 2020; 12(6):491. https://doi.org/10.3390/pharmaceutics12060491
Chicago/Turabian StyleFantini, Adriana, Anna Demurtas, Sara Nicoli, Cristina Padula, Silvia Pescina, and Patrizia Santi. 2020. "In Vitro Skin Retention of Crisaborole after Topical Application" Pharmaceutics 12, no. 6: 491. https://doi.org/10.3390/pharmaceutics12060491