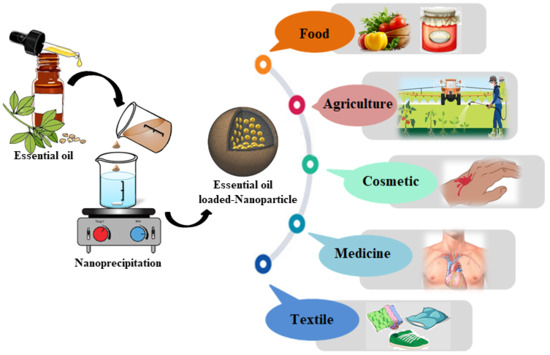
Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects
Abstract
:1. Introduction
2. Essential Oils
2.1. Chemical Composition of Essential Oils
2.2. Challenges in Rational Use of Essential Oils
3. Polymeric Nanoparticle
4. Nanoprecipitation Process to Encapsulate Essential Oils
4.1. Principle
4.2. Mechanism of Nanoparticle Formation
4.3. Raw Materials
4.3.1. The Solvent Phase
4.3.2. The Non-Solvent Phase
4.4. Physicochemical Properties of Nanoparticles Produced by Nanoprecipitation
| Essential Oil | Source of Essential oil | Part of the Plant | Solvent Phase | Non Solvent Phase | Biological Properties | Application | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymer | Surfactant | Solvent | Surfactant | Solvent | Size (nm) | Z. Pot (mV) | EE (%) | ||||||
| Palmarosa | Cymbopogon martini Roxb. | Leaves | PCL | Lecithin | Acetone | Pluronic F68 | Water | 282.1 | −27.2 | 99.54 | Antioxidant Antimicrobial | Cosmetic | [65] |
| Thyme | Thymus vulgaris L. | Stem + leaves | Eudragit®L100-55 | / | Acetone: Isopropanol | PVA | Water | 153.9 | −4.11 | 52.81 | Antioxidant | Food | [68] |
| Thymus serpyllum L. | Stem + leaves | Chitosan | / | Acetic acid | / | Methanol | / | / | 68 | Antimicrobial | Agriculture | [73] | |
| Chitosan | / | Acetic acid | / | Methanol | 117–226 | +27 | / | Antimicrobial | Agriculture | [81] | |||
| Thymus leptobotrys L. | Aerial part | Eudragit RS 100 | / | Ethanol | / | Water | 144 | +80.9 | / | Bacteriostatic Fungistatic | Medicine | [75] | |
| Thymus satureoides L. | Aerial part | Eudragit RS 100 | / | Ethanol | / | Water | 132 | 81.6 | / | Bacteriostatic Fungistatic | Medicine | [75] | |
| Bergamot | Citrus bergamia Risso. | Peels of fruit | Eudragit®RS100 | / | Acetone | / | Water | 57 to 208 | 39 to 74 | 28–84 | Antimicrobial | Food | [69] |
| Sweet orange | Citrus sinensis L. | Peels of fruit | Eudragit®RS100 | / | Acetone | / | Water | 57 to 208 | 39 to 74 | 56–96 | Antimicrobial | Food | [69] |
| Oregano | Origanum Vulgare L. | Leaves | PCL | Span 80 | Acetone | Tween 80 | Water | 181.6 | −40.7 | 85.9 | Antimicrobial | Textile | [95] |
| Rosemary | Rosmarinus officinalis L. | Aerial parts | Eudragit®EPO | / | Acetone: Isopropanol | / | Water | 200 | / | 59 | Antioxidant | Cosmetic | [70] |
| Leaves | PCL | Span 20 | Acetone | Tween 80 | Water | 145 | −11 | 78.2 | Insecticide | Agriculture | [93] | ||
| PCL | Span 20 | Acetone | Tween 80 | Water | 220 | −19.9 | 99 | Antioxidant Analgesic Antimicrobial | Medicine | [66] | |||
| Lavender | Lavandula dentata L. | Aerial parts | Eudragit®EPO | / | Acetone: isopropanol | / | Water | 200 | / | 41 | Antioxidant | Cosmetic | [70] |
| PEO-B-PLA | / | Acetone | / | Water | 10–75 | / | 70–75 | Antimicrobial Sedative | Textile | [88] | |||
| Nigella | Nigella sativa L. | Seeds | PCL | / | Acetone | PVA Tween 80 | Water | 230–260 | −30 to −20 | / | Anti-inflammatory | Cosmetic | [90,96] |
| Peppermint | Mentha piperita L. | Aerial parts | Cellulose acetate | / | Acetone | / | Water | 180 | −38 | / | Antimicrobial | Medicine | [71] |
| Cinnamon | Cinnamomum Cassia presl. | Bark | Cellulose acetate | / | Acetone | / | Water | 150 | −40 | / | Antimicrobial | Medicine | [71] |
| Lemongrass | Cymbopogon citratus DC. | Leaves | Cellulose acetate | / | Acetone | / | Water | 200 | −36 | / | Antimicrobial | Medicine | [71] |
| Leaves | PLA | / | Acetone | / | Water | 300 | −6 | / | Antimicrobial | Medicine | [55] | ||
| Pepper tree | Shinus mole L. | Leaves | Chitosan | / | Acetic acid | / | Methanol | 355.3 | / | / | Antifungal | Food | [72] |
| Leaves | Chitosan | / | Acetic acid | / | Methanol | 754 | +9.1 | / | Antifungal | Agriculture | [74] | ||
| Lime | Citrus aurantiifolia Christm. | Peels of fruit | Chitosan | / | Acetic acid | / | Methanol | / | +10 | / | Antimicrobial | Food | [82] |
| Peels of fruit | Chitosan | / | Acetic acid | / | Methanol | 250 | +10 | / | Antimicrobial | Agriculture | [81] | ||
| Geraniol | / | / | PluronicF-127 | / | THF | / | Water | 26–412 | / | / | Antimicrobial | Food | [86] |
| PCL | Lecithin | Acetone | Pluronic F68 | Water | 289.3 | −26.6 | 99.88 | Antioxidant Antimicrobial | Cosmetic | [65] | |||
| Zanthoxylum rhoifolium | Zanthoxylum rhoifolium L. | Leaves | PCL | Span 60 | Acetone | Tween 80 | Water | ˂500 | −20 | 96 | Pesticide | Agriculture | [87] |
| Pelargonium graveolens | Pelargonium graveolens L’Hér. | Aerial part | Eudragit RS 100 | / | Ethanol | / | Water | 113 | +80.6 | / | Bacteriostatic Fungistatic | Medicine | [75] |
| Eugenia Caryophyllata | Eugenia Caryophyllata C. | Buds | Eudragit RS 100 | / | Ethanol | / | Water | 131 | +80.7 | / | Bacteriostatic Fungistatic | Medicine | [75] |
| Carvone | / | / | PLGA | / | DMS | / | Water | 126 | / | 61 | Antimicrobial | Food | [67] |
| Anethole | / | / | PLGA | / | DMS | / | Water | 158 | / | 87 | Antimicrobial | Food | [67] |
| Thymol | / | / | Ethyl cellulose Methyl cellulose | / | Ethanol | / | Water | 420 | / | 77 | Antimicrobial | Cosmetic | [97] |
| Carvacrol | / | / | PLGA | Epikuron 200 | acetone | Pluronic F68 | Water | 209 | −19 | 26 | Antimicrobial | Medicine | [57] |
5. Applications
5.1. Agriculture Field
5.2. Food Field
5.3. Medicinal Field
5.4. Cosmetic Field
5.5. Textiles
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Heiss, S.S.H.; Rollinger, J.M.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, I.; Shuid, A.; Borhanuddin, B.; Fozi, N. The application of phytomedicine in modern drug development. Internet J. Herb. Plant. Med. 2012, 1, 2. [Google Scholar]
- Robinson, M.M.; Xiaorui, Z. The World Medicines Situation (Traditional Medicines: Global Situation, Issues Andchallenges); World Heal Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- El-Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of essential oils and factors influencing their constituents. In Soft Chemistry and Food Fermentation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2017; pp. 379–419. [Google Scholar]
- Pires, V.P.; Almeida, R.N.; Wagner, V.M.; Lucas, A.M.; Vargas, R.M.F.; Cassel, E. Extraction process of the Achyrocline satureioides (Lam) DC. essential oil by steam distillation: Modeling, aromatic potential and fractionation. J. Essent. Oil Res. 2019, 31, 286–296. [Google Scholar] [CrossRef]
- Vega, A.F.; Corona, N.R.; Palou, E.; Malo, A.L. Estimation of mass transfer coefficients of the extraction process of essential oil from orange peel using microwave assisted extraction. J. Food Eng. 2016, 170, 136–143. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Nikmaram, N.; Esteghlal, S.; Khaneghah, A.M.; Niakousari, M.; Barba, F.J.; Roohinejad, S.; Koubaa, M. Efficiency of ohmic assisted hydrodistillation for the extraction of essential oil from oregano (Origanum vulgare subsp. viride) spices. Innov. Food Sci. Emerg. Technol. 2017, 41, 172–178. [Google Scholar] [CrossRef]
- Hernández, L.A.C.; Victoria, J.R.E.; Trejo, A.; Beltrán, J.Á.G. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus Off. Spenn.). J. Food Eng. 2017, 200, 81–86. [Google Scholar]
- Sodeifian, G.H.; Sajadian, S.A.; Ardestani, N.S. Optimization of essential oil extraction from Launaea acanthodes Boiss.: Utilization of supercritical carbon dioxide and cosolvent. J. Supercrit. Fluids 2016, 116, 46–56. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Mihai, A.L.; Popa, M.E. Essential oils utilization in food industry—A literature review. Sci. Bull. Ser. F Biotechnol. 2013, 17, 187–192. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; MacHial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety—E-Book: A Guide for Health Care Professionals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Shukla, A.C. Essential oils as green pesticides for postharvest disease management. Acta Hortic. 2018, 1210, 199–206. [Google Scholar] [CrossRef]
- Debonne, E.; van Bockstaele, F.; Samapundo, S.; Eeckhout, M.; Devlieghere, F. The use of essential oils as natural antifungal preservatives in bread products. J. Essent. Oil Res. 2018, 30, 309–318. [Google Scholar] [CrossRef]
- Preedy, V.R. Essential Oils in Food Preservation, Flavor and Safety; Elsevier Science Publishing Co Inc.: New York, NY, USA, 2016. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pedro, A.S.; Santo, I.E.; Silva, C.V.; Detoni, C.; Albuquerque, E. The use of nanotechnology as an approach for essential oil-based formulations with antimicrobial activity. In Microbial Pathogens and Strategies for Combating Them: Science, Technology And Education; Méndez-Vila, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 2, pp. 1364–1374. [Google Scholar]
- Almeida, K.B.; Araujo, J.L.; Cavalcanti, J.F.; Romanos, M.T.V.; Mourão, S.C.; Amaral, A.C.F.; Falcão, D.Q. In vitro release and anti-herpetic activity of Cymbopogon citratus DC. volatile oil-loaded nanogel. Rev. Bras. Farm. 2018, 28, 498–502. [Google Scholar]
- Choi, M.; Soottitantawat, A.; Nuchuchua, O.; Min, S.; Ruktanonchai, U. Physical and light oxidative properties of eugenol encapsulated by molecular inclusion and emulsion-diffusion method. Food Res. Int. 2009, 42, 148–156. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, F.; Li, X.; Zhang, X.; Abbas, S. Formation of heat-resistant nanocapsules of jasmine essential oil via gelatin/gum arabic based complex coacervation. Food Hydrocoll. 2014, 35, 305–314. [Google Scholar] [CrossRef]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From particle preparation to in vivo applications. In Polymer Nanoparticles for Nanomedicines; Vauthier, C., Ponchel, G., Eds.; Springer: Cham, Switzerland, 2016; pp. 17–53. [Google Scholar]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo-Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- AFNOR. Huiles Essentielles, Tome 2, Monographies Relatives Aux Huiles Essentielles, 6th ed.; AFNOR, Association Francaise de Normalisation: Paris, France, 2000. [Google Scholar]
- European Pharmacopeia. European Directorate for the Quality of Medicines and Health Care, 9th ed.; European Pharmacopeia: Strasbourg, France, 2018. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Faleiro, M. The mode of antibacterial action of essential oils. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1143–1156. [Google Scholar]
- Cava, R.; Nowak, E.; Taboada, A.; Marin-Iniesta, F. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. J. Food Prot. 2007, 70, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shushni, M.A.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Damte, D.; Lee, S.; Kim, J.; Park, S. Antimicrobial activity of lemongrass and oregano essential oil against standard antibiotic resistant Staphylococcus aureus and field isolates from chronic mastitis cow. Int. J. Phytomed. 2012, 4, 134–139. [Google Scholar]
- Mathlouthi, N.; Bouzaienne, T.; Oueslati, I.; Recoquillay, F.; Hamdi, M.; Urdaci, M.; Bergaoui, R. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: In vitro antimicrobial activities and effects on growth performance. J. Anim. Sci. 2012, 90, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Lysakowska, M.; Denys, P.; Kowalczyk, E. The antimicrobial activity of thyme essential oil against multidrug resistant clinical bacterial strains. Microb. Drug Resist. 2012, 18, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential oils. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 3–17. [Google Scholar]
- Caldefie-Chézet, F.; Fusillier, C.; Jarde, T.; Laroye, H.; Damez, M.; Vasson, M. Potential antiinflammatory effects of Malaleuca alternifolia essential oil on human peripheral blood leukocytes. Phyther. Res. 2006, 20, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Pearce, A.; Marshman, G.; Finlay-Jones, J.; Hart, P. Tea tree oil reduces histamine-induced skin inflammation. Br. J. Derm. 2002, 147, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.; Alviano, A.; Blank, A.; Alves, P.; Alviano, C.; Gattass, C. Melisa officinalis L. essential oil: Antitumoral and antioxidant activities. J. Pharm. Pharm. 2004, 56, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Peng, Z.; Fu, X.; Liu, C.; Xu, Y.; Ji, W.; Fan, J.; Chen, L.; Fang, L.; Huang, Y.; et al. Volatile oil from Saussurea lappa L. exerts antitumor efficacy by inhibiting epithelial growth factor receptor tyrosine kinase-mediated signaling pathway in hepatocellular carcinoma. Oncotarget 2016, 7, 79761–79773. [Google Scholar] [CrossRef] [Green Version]
- Tilaoui, M.; Mouse, H.A.; Jaafari, A.; Aboufatima, R.; Abderrahman, C.; Zyad, A. Chemical composition and antiproliferative activity of essential oil from aerial parts of a medicinal herb Artemisia herba-alba Asso. Rev. Bras. Farm. 2011, 21, 781–785. [Google Scholar] [CrossRef] [Green Version]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Salvatore, G.; MondeIlo, F.; Arancia, G.; Molinari, A. Terpinen-4-ol, the main component of Melaieuca aitemifolia L. (tea tree) oil inhibits the in vitro growth of human melanoma cells. J. Invest. Derm. 2004, 122, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Sylvestre, M.; Pichette, A.; Lavoie, S.; Longtin, A.; Legault, J. Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrine L. coulter. Phyther. Res. 2007, 6, 536–540. [Google Scholar] [CrossRef]
- Ríos, N.; Stashenko, E.E.; Duque, J.E. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae). Rev. Bras. Entomol. 2017, 61, 307–311. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Nouri-Ganbalani, G.; Hoseini, S.A.; Sadeghi, G.R. Insecticidal activity of essential oils of five aromatic plants against Callosobruchus maculatus F. (Coleoptera: Bruchidae) under laboratory conditions. J. Essent. Oil Bear. Plants 2012, 15, 256–262. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Hădărugă, N.G.; Costescu, C.I.; David, I.; Gruia, A.T. Thermal and oxidative stability of the Ocimum basilicum L. essential oil/β-cyclodextrin supramolecular system. Beilstein J. Org. Chem. 2014, 10, 2809–2820. [Google Scholar]
- Bogusz, M.J.; Al-Tufail, M. Chapter 18 toxicological aspects of herbal remedies. In Handbook of Analytical Separations; Hempel, G., Ed.; Elsevier: Oxford, UK, 2008; Volume 6, pp. 589–610. [Google Scholar]
- Sarigiannis, D.; Karakitsios, S.; Gotti, A.; Liakos, I.; Katsoyiannis, A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Sköld, M.; Karlberg, A.-T.; Matura, M.; Börje, A. The fragrance chemical β-caryophyllene-air oxidation and skin sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Sagiri, S.S.; Anis, A.; Pal, K. A review on encapsulation of vegetable oils: Strategies, preparation methods and applications. Polym. Plast. Technol. Eng. 2016, 55, 37–41. [Google Scholar] [CrossRef]
- Liakos, I.L.; Grumezescu, A.M.; Holban, A.M.; Florin, I.; D’Autilia, F.; Carzino, R.; Bianchini, P.; Athanassiou, A. Polylactic acid-lemongrass essential oil nanocapsules with antimicrobial properties. Pharmaceuticals 2016, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT Food Sci. Technol. 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Iannitelli, A.; Grande, R.; di Stefano, A.; di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly (DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef]
- Qiu, C.; Chang, R.; Yang, J.; Ge, S.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Preparation and characterization of essential oil-loaded starch nanoparticles formed by short glucan chains. Food Chem. 2017, 221, 1426–1433. [Google Scholar] [CrossRef]
- Paula, H.C.B.; Sombra, F.M.; Abreu, F.O.M.S.; Paula, R.C.M. Lippia sidoides essential oil encapsulation by angico gum/chitosan nanoparticles. J. Braz. Chem. Soc. 2010, 21, 2359–2366. [Google Scholar] [CrossRef] [Green Version]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]
- Zohri, M.; Gazori, T.; Mirdamadi, S.; Asadi, A.; Haririan, I. Polymeric nanoparticles: Production, applications and advantage. Internet J. Nanotechnol. 2009, 3, 217–223. [Google Scholar]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Froiio, F.; Mosaddik, A.; Morshed, M.T.; Paolino, D.; Fessi, H.; Elaissari, A. Edible polymers for essential oils encapsulation: Application in food preservation. Ind. Eng. Chem. Res. 2019, 58, 46. [Google Scholar] [CrossRef]
- González, J.O.W.; Jesser, E.N.; Yeguerman, C.A.; Ferrero, A.A.; Band, B.F. Polymer nanoparticles containing essential oils: New options for mosquito control. Environ. Sci. Pollut. Res. 2017, 24, 17006–17015. [Google Scholar] [CrossRef] [PubMed]
- Jummes, B.; Sganzerla, W.G.; da Rosa, C.G.; Noronha, C.M.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Antioxidant and antimicrobial poly-ε-caprolactone nanoparticles loaded with Cymbopogon martinii Roxb. essential oil. Biocatal. Agric. Biotechnol. 2020, 23, 101499. [Google Scholar] [CrossRef]
- Ephrem, E.; Greige-Gerges, H.; Fessi, H.; Charcosset, C. Optimisation of rosemary oil encapsulation in polycaprolactone and scale-up of the process. J. Microencapsul. 2014, 31, 746–753. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Ghaedi, Z.; Asemi, M.; Khanavi, M.; Manayi, A.; Jamalifar, H.; Atyabi, F.; Dinarvand, R. Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J. Pharm. Res. 2013, 7, 290–295. [Google Scholar] [CrossRef]
- Pina-Barrera, A.M.; Alvarez-Roman, R.; Baez-Gonzalez, J.G.; Amaya-Guerra, C.A.; Rivas-Morales, C.; Gallardo-Rivera, C.T.; Galindo-Rodriguez, S.A. Application of a multisystem coating based on polymeric nanocapsules containing essential oil of Thymus vulgaris L. to increase the shelf life of table grapes (Vitis vinifera L.). IEEE Trans. Nanobiosci. 2019, 18, 549–557. [Google Scholar] [CrossRef]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential oils-loaded polymer particles: Preparation, characterization and antimicrobial property. Polymers 2019, 11, 1017. [Google Scholar] [CrossRef] [Green Version]
- Silva-Flores, P.G.; Opez, L.A.P.-L.; Rivas-Galindo, V.M.; Paniagua-Vega, D.; Galindo-Rodrıguez, S.A.; Alvarez-Roman, R. Simultaneous GC-FID quantification of main components of Rosmarinus officinalis L. and Lavandula dentata L. essential oils in polymeric nanocapsules for antioxidant application. J. Anal. Methods Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M.; Mihai, A.; Maria, A. Cellulose acetate-essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Chavez-Magdaleno, M.E.; Luque-Alcaraz, A.G.; Gutierrez-Martınez, P.; Cortez-Rocha, M.O.; Burgos-Hernandez, A.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Effect of chitosan-pepper tree (Schinus molle L.) essential oil biocomposites on the growth kinetics, viability and membrane integrity of colletotrichum gloeosporioides. Rev. Mex. Ing. Química 2018, 17, 29–45. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.; Bautista-Banos, S.; Gómez, Y.G. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Luque-Alcaraz, A.G.; Cortez-Rocha, M.O.; Velázquez-Contreras, C.A.; Acosta-Silva, A.L.; Santacruz-Ortega, H.D.C.; Burgos-Hernández, A.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Enhanced antifungal effect of chitosan/pepper tree (Schinus molle L.) essential oil bionanocomposites on the viability of Aspergillus parasiticus spores. J. Nanomater. 2016, 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- El-Asbahani, A.; Miladi, K.; Addi, H.; Bitar, A.; Casabianca, H.; Abdelhamid, E.M.; Hartmann, D.; Jilale, A.; Renaud, F.; Elaissari, A. Antimicrobial activity of nano-encapsulated essential oils: Comparison to non-encapsulated essential oils. J. Colloid Sci. Biotechnol. 2015, 4, 39–48. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Flores, F.C.; De Lima, J.A.; Ribeiro, R.F.; Alves, S.H.; Rolim, C.M.B.; Beck, R.C.R.; Silva, C.B. Antifungal activity of nanocapsule suspensions containing tea tree oil on the growth of Trichophyton rubrum. Mycopathologia 2013, 175, 281–286. [Google Scholar] [CrossRef]
- Li, K.; Yin, S.; Ynag, X.; Tang, C.; Wei, Z. Fabrication and characterization of novel antimicrobial films derived from thymol-loaded zein–sodium caseinate (SC) nanoparticles. J. Agric. Food Chem. 2012, 60, 11592–11600. [Google Scholar] [CrossRef]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2011, 76, 16–24. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Valverde-Aguilar, G.; Plascencia-Jatomea, M.; Correa Pacheco, Z.; Jiménez-Aparicio, A.; Solorza-Feria, J.; Barrera-Necha, L.; Bautista-Baños, S. Characterization of chitosan nanoparticles added with essential oils. In vitro effect on Pectobacterium carotovorum. Rev. Mex. Ing. Química 2015, 14, 589–599. [Google Scholar]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT Food Sci. Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–123. [Google Scholar] [CrossRef]
- Sugimoto, T. Preparation of monodispersed colloidal particles. Adv. Colloid Interface Sci. 1987, 28, 65–108. [Google Scholar] [CrossRef]
- Belda-Galbis, C.M.; Pina-Pérez, M.C.; Leufvén, A.; Martínez, A.; Rodrigo, D. Impact assessment of carvacrol and citral effect on Escherichia coli K12 and Listeria innocua growth. Food Control. 2013, 33, 536–544. [Google Scholar] [CrossRef]
- Yegin, Y.; Perez-Lewis, K.L.; Zhang, M.; Akbulut, M.; Taylor, T.M. Development and characterization of geraniol-loaded polymeric nanoparticles with antimicrobial activity against foodborne bacterial pathogens. J. Food Eng. 2016, 170, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Christofoli, M.; Costa, E.C.C.; Bicalho, K.U.; de Cássia-Domingues, V.; Peixoto, M.F.; Alves, C.C.F.; Araújo, W.L.; de Melo-Cazal, C. Insecticidal effect of nanoencapsulated essential oils from Zanthoxylum rhoifolium L. (Rutaceae) in Bemisia tabaci populations. Ind. Crop. Prod. 2015, 70, 301–308. [Google Scholar] [CrossRef]
- Popiolski, T.M.; Otsuka, I.; Halila, S.; Muniz, E.C.; Soldi, V.; Borsali, R.; Catarina, F.D.S. Preparation of polymeric micelles of poly (ethylene oxide-b-lactic acid) and their encapsulation with lavender oil. Mater. Res. 2016, 19, 1356–1365. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus Aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Badri, W.; El Asbahani, A.; Miladi, K.; Baraket, A.; Agusti, G.; Agha, Q.; Errachid, A.; Fessi, H.; Elaissari, A. Poly (ε-caprolactone ) nanoparticles loaded with indomethacin and Nigella Sativa, L. essential oil for the topical treatment of inflammation. J. Drug Deliv. Sci. Technol. 2018, 46, 234–242. [Google Scholar] [CrossRef]
- Shakeri, F.; Shakeri, S.; Hojjatoleslami, M. Preparation and characterization of carvacrol loaded polyhydroxybutyrate nanoparticles by nanoprecipitation and dialysis methods. J. Food Sci. 2014, 79, 697–705. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/microspheres: Preparation, physicochemical characterization, and storage stability. Ind. Crop. Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Khoobdel, M.; Ahsaei, S.M.; Farzaneh, M. Insecticidal activity of polycaprolactone nanocapsules loaded with Rosmarinus officinalis L. essential oil in Tribolium castaneum (Herbst). Entomol. Res. 2017, 47, 175–184. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.Á.; Hernández-López, M. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv hass avocado and fruit quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N. Antimicrobial finishing of cotton and polyamide with nano-microparticles. In Proceedings of the International Conference of Applied Research On Textile, CIRAT-8, Monastir, Tunisia, 9–10 November 2018; pp. 2286–5659. [Google Scholar]
- Badri, W.; Mohamed, F.; Affendi, M.; Asbahani, A.; Miladi, K.; Nazari, Q.; Viennet, C.; Robin, S.; Fessi, H.; Elaissari, A. Topical co-delivery of indomethacin and Nigella sativa L. essential oil in poly-caprolactone nanoparticles: In vivo study of anti-inflammatory activity. Int. J. Adv. Res. 2018, 6, 801–816. [Google Scholar] [CrossRef] [Green Version]
- Wattanasatcha, A.; Rengpipat, S.; Wanichwecharungruang, S. Thymol nanospheres as an effective anti-bacterial agent. Int. J. Pharm. 2012, 434, 360–365. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int Soc. Prev. Community Dent. 2015, 5, 338–340. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora L. essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. Food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Hamdi, M.; Restuccia, C. Efficacy of the combined application of chitosan and locust bean gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int. J. Food Microbiol. 2014, 170, 21–28. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Choudhary, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Zhu, D.; Chenga, H.; Li, J.; Zhang, W.; Shen, Y.; Chen, S.; Ge, Z.; Chen, S. Enhanced water-solubility and antibacterial activity of novel chitosan derivatives modified with quaternary phosphonium salt. Mater. Sci. Eng. C 2016, 61, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Padilla, A.; Soto, K.M.; Mendoza, S. Food antimicrobials nanocarriers. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Mack, D.; Rohde, H.; Harris, L.; Davies, A.; Horstkotte, M.; Knobloch, J. Biofilm formation in medical device-related infection. Int. J. Artif. Organs 2006, 29, 343–359. [Google Scholar] [CrossRef]
- Jousset, A.; Dortet, L.; Naas, T. Multidrug resistant bacteria and emerging antibiotic resistance traits. Rev. Prat. 2017, 67, 211–217. [Google Scholar]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Komplementmed 2009, 16, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.X.; Chen, S.L. Aromachology and its application in the textile field. Fibres Text. East. Eur. 2005, 13, 41–44. [Google Scholar]
- Andonova, V. Synthetic polymer-based nanoparticles: Intelligent drug delivery systems. In Acrylic Polymers in Healthcare; Reffy, B.S.R., Ed.; Intech Open: Madrid, Spain, 2017; pp. 101–125. [Google Scholar]
- Martín, Á.; Varona, S.; Navarrete, A.; Cocero, M.J. Encapsulation and co-precipitation processes with supercritical fluids: Applications with essential oils. Open Chem. Eng. J. 2010, 4, 31–41. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural polymers: Polysaccharides and their derivatives for biomedical applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Newnes: Oxford, UK, 2014; pp. 67–89. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. https://doi.org/10.3390/pharmaceutics12050431
Lammari N, Louaer O, Meniai AH, Elaissari A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics. 2020; 12(5):431. https://doi.org/10.3390/pharmaceutics12050431
Chicago/Turabian StyleLammari, Narimane, Ouahida Louaer, Abdeslam Hassen Meniai, and Abdelhamid Elaissari. 2020. "Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects" Pharmaceutics 12, no. 5: 431. https://doi.org/10.3390/pharmaceutics12050431